There’s a bunch of Latin and Greek in botany. I’m pretty intimidated by all the scientific names for species, and even families. The words for a lot of the kinds of things I’m interested in right now, that is of structures, relationships, and behaviors, are much more tractable. A lot of words like «stem» or «flower» might feel familiar, but oddly enough, I’m finding terms that seem intimidating are more revealing in their ability to compose with other intimidating seeming words.
As an example of this «phyllotaxy» looks intimidating. But if you knew that «phyllo-» meant «leaves» and «-taxy»/»-taxis» meant «arrangement,» you’d be good to go. And if you came upon «rhizotaxy» later, you’d at least know it meant the arrangement of something. (spoiler for later in this list, «rhizo» means «root»).
I recently got A Botanist’s Vocabulary as a gift. It’s very good, but the organization (the alphabet) doesn’t quite work how I’d like. The cross references for related and synonymous terms (and the illustrations) are helpful, but an organization that gives a few more related categories for words and parts of words is something else I’m keeping track of. This post is to note the technical terms that mostly seem to combine well and often.
Before the useful words, a brief note on how this continues the purpose outlined in previous posts. Entrepreneurial/business thinking is an invasive species. I don’t want to turn plant vocabulary along with its models and lessons into software because everything should be thought of in terms of software. This is overall a horticultural project of putting these metaphors, models, and stories into the cracks between the asphalt in our heads. Learning these words is a path toward bewilderment and promoting a broader sense of flourishing than the economic common sense that has been beaten into us.
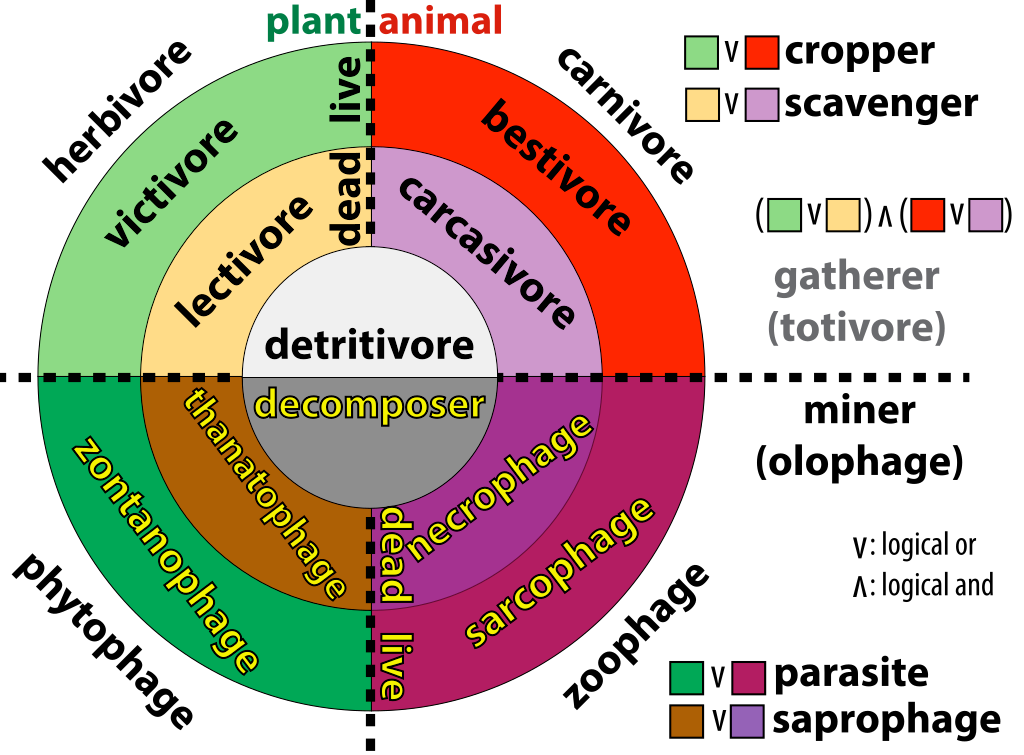
Eating
-vore
x-eater. Examples you might know are:
- carnivore: eating animals
- herbivore: eating plants
But here’s a broader view called the Getz Diagram.
-phage
Woah now. What’s with this «- phage» suffix? I guess the distinction is whether something is a gatherer (a «totivore») or a miner (an «olophage»). I don’t know where you draw the distinction here, but generally gatherers do more moving around to get their food. «x-vory» (eating x) and «y-phagy» (eating y) seem like squishy distinctions in some cases, but anyways, here’s a couple of words that use those suffixes:
- Frugivory: Eating fruit
- Geogphagia: Eating rocks/dirt/earth
So what’s cool is if you know half the word, you can probably guess at some more. If frugivory is eating fruit, and you know «avi-» is the prefix for birds, well then bam, you can probably guess an avivore eats birds. Good stuff.
Anyways, here are some birds eating dirt.
I suspect my guesses of what is a «-vore» and what is a «-phage» would get better with more examples, but if I see either one, I know a thing is being eaten. Good stuff.
-troph
«Trophic levels» are what link in a foodchain/foodweb an organism fits, but if you see «-trophy» or «-troph» it’s more about nutrition/consumption in a more general way than «eating.» Here are a couple you might see:
- Autotroph: Obtaining energy from an inorganic source, like sunlight (aka photoautotrophy).
- Heterotroph: Obtaining energy from other organisms.
Incidentally, there are autotrophs that aren’t photoautotrophs and plants that are heterotrophic, like the mycoheterotrophic (fungus eating) ghost plant.
Why is the ghost plant «mycoheterotrophic» and not «mycoheterophagic» or «mycoheterovorous?» I don’t know. If I saw any of the three I would know what it meant, and if I said any of the wrong ones to a real and kind botanist, they’d set me straight. Good enough for me, for now.
Self and Other
And just like that, we stumbled onto our next kind of words of interest: «auto» (self) and «hetero» (other/different). Let’s toss in «homo» (same/self) and «allo» (other) while we’re at it.
Auto-
- Autogamy: self-pollination
- Autochory: dispersal of seeds without any
Allo-
- Allogamy: cross-pollination
- Allopatric: having a population in multiple areas
Hetero-
- Heterophyllus: having leaves of a different shape
- Heterogamous: having distinct male and female flowers
Homo-
- Homology: having a structure common to two ancestors like gorilla arms and human arms (contrasted with «analogy» like bird and insect wings, where both are created to solve the problem of flight, but don’t share a common ancestry where the structure developed. This is also called «convergent evolution»/»convergence»)
- Homochromous: being all of the same color
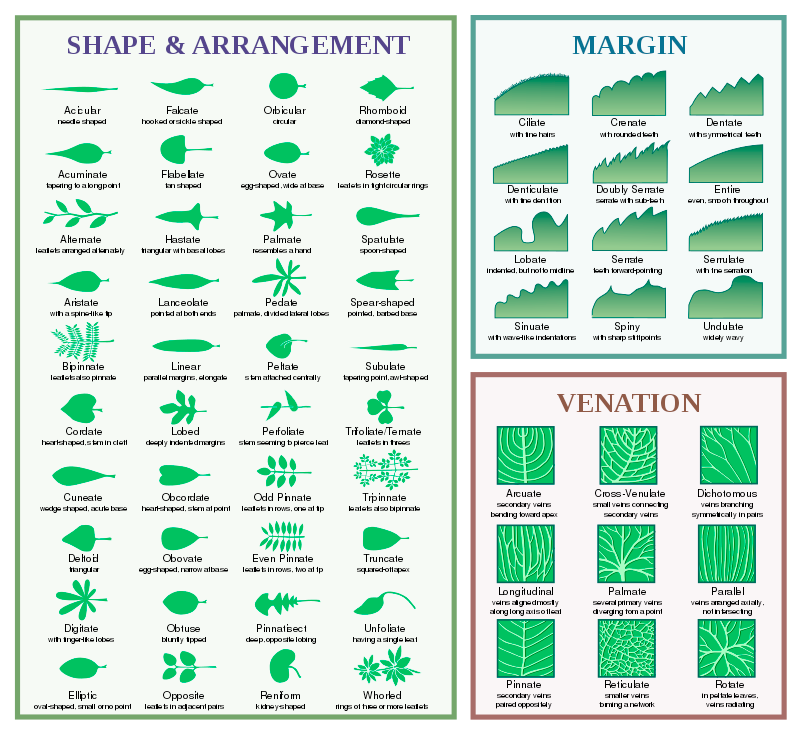
Plant Shapes and Parts
If you’re looking for a list of words for plant parts, this glossary of plant «morphology» has a good larger list. Before getting too much further, «-phyte,» «-phytic,» and «phyto-» should be noted to mean «plant.» It comes up a lot!
-phyte/-phytic
- epiphyte: a plant that grows on other plants
- epiphytic: growing on plants
phyto-
- phytogenesis: growth and development of plants
-morph- (shape)
- morphology: the study of shapes/forms
- xeromorphic: structures adapted to dry climates
There are also a couple of words that are too important to worry about how they combine with others.
- fruit: the thing with the seed(s). What we call «nuts» are usually fruits or parts of fruits. Tomatoes are fruits. Cucumbers are fruits. Olives? Oh boy you better believe they’re fruits.
- vegitative parts: non-reproductive parts of a plant
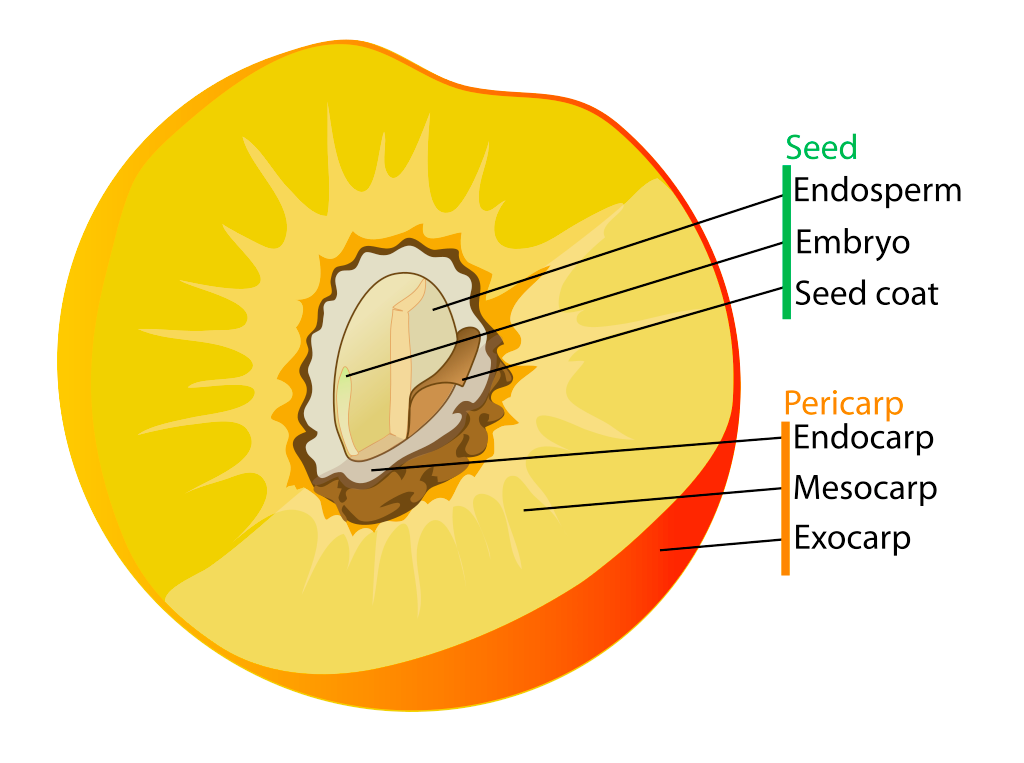
Speaking of fruits, -carp is the suffix that means fruit. And a few location/relation type words (peri-, endo-, meso-, exo-) are good for talking about the tasty parts of stone fruits (like peaches or avacados).
peri- (about/around)
- pericarp: fruit part that surrounds the seed
- peripterous: surrounded by a wing
endo- (center)
- endocarp: the part of the fruit closest to the seed
meso- (middle)
- mesocarp: the middle part of a pericarp
- mesophyte: plants that aren’t adapted to a specifically wet or dry environment
exo- (outer)
- exocarp: the outer part of a pericarp (eg. «skin» of an apple)
epi- (on)
- epicarp: same as exocarp
- epilithic: growing attached to rock
-a (not)
- apetalous: not having petals
- achlorophyllous: not having chlorophyll
- abiotic: the non-living parts of an ecosystem
-phyll (leaf)
- triphyllous: having three leaves
- epiphyllous: attached to the leaf of a plant
- chlorophyll: the pigment in green leaves
clad- (branch)
- cladogram: a branching diagram used to describe the ancestry of organisms
- cladophyll: stem that looks and functions like a leaf
- phylloclade: cladophyll
rhizo- (mass of roots)
- rhizome: an underground stem that sends out shoots, roots, and maybe produces tubers
- rhizotaxy: the arrangement of roots
pheno- (show)
- phenotype: the observable characteristics and traits in an organism (also differentiation within a species)
- phenology: the study of observable lifecycle events (eg. leaves falling, flowers blooming, etc)
Lifecycle and Evolution
You might have found some linguistics mistakes already. If you’re worried I’m going to do it again, strap in. I’m about to fuck up:
-gene/genetic (also «gonos» for offspring and procreation)
- phylogenetic (tree): the evolutionary tree (may be drawn as a cladogram, but there are other interesting options)
-geny/-genic/ (production)
- phylogeny: the history of the evolution of an organism
- ontogeny: the study of an organism’s lifespan
By the way, goofballs used to think that phylogeny and ontogeny (evolution and lifecycle) were connected in a weird way. I guess they aren’t, and human embryos don’t go through a fish phase, lizard phase, and hamster phase in the womb (or whatever the recapitulation theory says that’s probably less fun but more reasonable on its face).
Ok. So some things might happen in a plant’s life. They might produce things, which can be described as -ferous
-ferous
- coniferous: producing cones
- nectariferous: producing nectar
- frugiferous: producting fruit
- oviferous: producing eggs
Next up is «-tropism.» This denotes a response (slowish movement/growth) to some stimulus (faster movement, like with venus fly traps, is called… «rapid plant movement»). Don’t get «-tropic» and «-trophic» mixed up. The second one is about eating. This one is about moving.
-tropic
- hydrotropism: movement or growth in response to water
- phototropism: movement or growth in response to light
- thigotropism: movement or growth in response to touch/contact
Study
-ology comes up a lot. The thing in front of it is sometimes new.
-ology (study)
- biology: study of living things
- mycology: study of fungi
- ecology: study of environment
- denrology: study of trees
-nomy (system)
- taxonomy: arrangement of systems
Note here that «taxon» (singular) or «taxa» (plural) is a way to refer to an individual point in a cladogram. As in, you might say «this taxon is [some species name]» or «this taxon is [some genus name]»
Seed Dispersal & Pollination
So the suffix for seed dispersal is -chory, and for pollination is -phily.
-chory
- allochory: seed dispersal with an external vector (wind, animal, etc.)
- autochory: seed dispersal without an external vector
- anemochory: seed dispersal by wind
- ballochory: seed dispersal by «explosive dehiscense (fruit opening)» of the fruit (check this out. And this)
- hydrochory: seed dispersal by water
- myrmechory: seed dispersal by ants (the seeds have an «elaisome» attached, which the ants feed to their larve and put the seed in their big ant trash pile, which is apparently a good germination site)
- anthropochory: seed dispersal by people
- chiropterochory: seed dispersal by bats
- malacochory: seed dispersal by molluscs
- ornithochory: seed dispersal by birds
- zoochory: seed dispersal by animals
- epizoochory: seed dispersal by animals (seeds travel on the outside of them, like burrs and foxtails)
- endozoochory: seed dispersal by animals (seeds travel inside of them, like if )
- diplochory: two-phase dispersal
-phily
- anemophily: pollination by wind
- hydrophily: pollination by water
- entomophily: pollination by insects
- chiropterophily: pollination by bats
- ornithophily: pollination by birds
- zoophily: pollination by animals
By the way, bees can do a thing called «buzz pollination» where they bite the anther (where the pollen comes out) and vibrate to shake it out.
Habitat
Sometimes it’s wet, sometimes it’s dry, and sometimes it’s in between.
xero- (dry)
- xerophyte: plants that grow in dry conditions
- xeric: dry habitats
- xeromorphic: having dry habitat adaptive structures
hydro- (water/wet)
- hydrophyte: plants that grow in wet conditions/water
- hydric: wet habitat
meso- (medium)
- mesophyte: plants that grow in conditions of moderate moisture
- mesic: habitat of moderate moisture
halo- (salt)
- halophyte: salt tolerant plants
aero- (air)
- aerophyte: plant that grows on another plant (also epiphyte)
eco-
- ecotone: transition area between two biomes
- ecocline: an area that denotes a population that has a particular trait (ecotype if multiple traits are affected)
- ecotope: smallest designations in ecologically distinct landscape features
Number
There are prefixes for fractional numbers too apparently. Unsurprisingly, Wikipedia has a big list. One through 12 all come up a lot. Here are a few usages:
uni- (one)
- unifoliate: having only one leaf
- unisexual: having only male or only female functional reproductive parts
mono- (one)
- monocot: plants that have one seed leaf and leaves that are parallel
- monotypic: a taxon with one type of a lower ranking (eg. a genus with only one species)
bi- (two)
- bicolored: having two colors
- bisexual: having both female (egg) and male (sperm) reproductive cells in the same organism or structure
- biennial: having a two year life cycle
di- (two)
- dicot: plants that have two seed leaves that are net-veined
- didymous: occuring in pairs
tri- (three)
- trifoliate: having three leaflets
- trimerous: having flower parts in multiples of three
- tripartite: divided into three parts
quadri- (four)
- quadrate: square/rectangular
- quadrifoliate: has 4 leaves
tetra- (four)
- tetramerous: having flower parts in multiples of four
- tetraploid: having 4 sets of chromosomes
And so on. Numbers just keep going.
That’s it.
There are a bunch more words, but these are the ones I’ve liked so far, and they’re not the typical things I remember from junior high biology. Have fun. Oh. And here’s a favorite plant of mine lately: lithops.
There are all sorts of words and phrases you will hear as you learn more about gardening, and the jargon can be overwhelming.
To help you soak up as much information as possible when reading about growing your own food or chatting with a more experienced gardener, here is your gardening vocabulary lesson.
It’s another one of those gardening cheatsheets (like this one about plant families!) that you will want to come back and revisit, because it’s chock full of information.
Plant Parts and Anatomy
Foliage: a word describing the leaves and/or branches of a plant
Types of Seeds
Here is a fantastic article explaining the differences between heirloom, hybrid and open-pollinated.
Plant Types Vocabulary
Annual: a plant that completes its entire life cycle in one year
Biennial: a plant that takes two years to complete its entire life cycle
Perennial: a plant that grows for more than two years
Cultivar: a variety of a plant developed through selective breeding. Most vegetables are cultivars as they have been selectively bred over generations to produce desirable characteristics
Starting Seeds Indoors
Dampen Off: a fungus that causes the stem to rot off at the soil level (be sure your seed starting containers are clean – if reusing, be sure to wash thoroughly between uses)
Harden off: slowly acclimating starts to the outside elements by placing them outside for a few hours one day, four hours the next day and progressing daily until they are ready to be outside all day and then planted outside to continue their life cycle
Rootbound: when a plant’s roots have outgrown the pot it is in and can no longer stretch and expand due to being trapped, or bound, inside the pot
Starts, or Transplants: immature plants that are often started inside in small trays before being put in the ground outside (these are the small plants you see for sale at garden stores)
Seedlings: the very first stage of a plant grown from seed, when the first stem/leaves start to emerge from the seed
Starting Seeds Outside
Broadcast: sprinkling down seeds without purposefully placing them in a spot
Direct Sow: to plant a seed outdoors, in the area that the plant will remain for its entire life cycle
Sow: a term used for planting seeds
Thin: the act of cutting plants at the soil level to allow the others plants near it to grow to maturity (e.g. if you sprinkle down an entire packet of kale seeds, you will need to thin some of them as they grow or there won’t be enough space for any to reach full size.)
Undersow: to plant seeds in an area that already has established seeds or crops – This is usually used in reference to sowing cover crops in the *understory of the vegetable crop, so that the cover crop can slowly get established before the vegetable crop is harvested. *understory: a layer of vegetation beneath the main canopy of the forest
Germination: when a seed first starts growing. This marks the end of a seed’s dormancy, or time of inactivity.
Growing Methods
Bareroot: plants that are dug up from the ground after they enter dormancy and are stored without any soil around their roots until replanted (strawberries are an example of a plant that can be purchased bareroot)
Cutting, or slip: to take part of the stem, roots or leaves of a mature plant and place it in potting soil to grow a new plant
Graft: tissues of two plants are joined together to make one new plant (e.g. You can graft multiple apple trees onto one tree, so the tree will produce many different kinds of apples on one tree.)
Propagate: to grow plants from seed, cuttings, or grafting
Weather Gardening Terms
Bolting: when a plant goes to seed due to increased daylight hours and/or high temperatures (the plant puts all of its energy into reproducing and stops putting energy into the edible parts of the plant, potentially causing them to become bitter)
Cool season crops: crops that grow in cool temperatures, usually in spring and fall, and bolt in high temperatures – more information and examples here
Frost date: dates for your average first and last freeze – find yours by heading here and clicking on Frost Calculator
Full sun: at least 6 hours of direct sunlight (many warm-season crops need 8-10 hours of direct sunlight)
Growing season: the time between the last average frost and the first average frost for your area (This is important when determining if a crop is right for your area because each crop has a certain number of days until it reaches maturity – usually found on the seed packet.)
Hard Freeze: when outside temperatures drop below 25°F for four, or more, hours (most plants need protection at 25°F or below)
Hardiness zone: a geographic zone based on the minimum temperatures of the region and the plants that can survive those temperatures
Light frost: temperature drops below 32°F (many cool season crops become sweeter after a light frost, such as kale and carrots)
Warm season crops: crops that germinate and grow in warm weather and will not survive a freeze (most do not do well under 50°F) – more information and examples of these crops here
Soil Terms
Cover crop: a plant whose purpose is enriching the soil with organic matter, preventing soil erosion or adding green manure to the soil
Mulch: an organic or inorganic material used to cover soil in order to hold in more moisture, eliminate weeds, regulate soil temperature and more – more information about types of mulch and its benefits here
Sheet mulch: layering thick pieces of organic material on top of soil to suppress weeds and build soil fertility – more specifics and a diagram here
Soil amendment: a material added to the soil with the purpose of making improvements (adding nutrients for plant life, retaining moisture, aerating, etc.)
Soil quality: how fertile a soil is. This is a general term describing how rich a soil is in nutrients and other beneficial factors
Humus: the dark organic material that makes up soil, composed of any variety of living matter such as decayed leaves, twigs, animal and insect matter
Compost: the product of decayed organic material, typically used in gardening as a fertilizer and soil amendment
Good drainage: soil that drains water well and doesn’t get easily over-saturated
Garden Helpers, Pests and Pest Control Words
Beneficials: organisms that help by pollinating plants, eating garden pests (e.g. ladybugs eat aphids) and/or parasitizing pests
Companion planting: plants that benefit others when grown next to them (by providing shade, deterring pests, etc.)
Crop rotation: the practice of rotating your crops so that the same plants are not grown in the same space for more than one season (This method is often used to manage pests in organic gardening.)
Pest: insects that cause harm to plants and/or eat your crops
Harvest Terminology
Cut and come again: you can cut a few leaves and the plant will continue to produce more, allowing you to harvest from one plant for an extended period of time (most greens, are cut and come again, such as lettuces and herbs)
Tomato Terms
Determinate: bushier tomato plants that have a shorter season and fruit that typically ripens all within a few weeks – more information and tips for growing tomatoes here.
Indeterminate: vining tomato plants with no set height that ripen fruit throughout the season and may grow until the first frost- more information and tips for growing tomatoes here.
Pruning: cutting, trimming, and removing plant parts for aesthetic reasons (to affect the plant shape and how it grows) or functional reasons (to help the plant focus on fruiting or developing more flower, for example).
Plant Reproduction
Dead-head: to remove dead blossoms in order to encourage more flower growth or prevent the plant from self-seeding
Self-fertile: a plant that does not need pollen from another plant to reproduce
Self-seed: when plants spread large amounts of their seed on their own
Gardening Tools
Hoe: a long-handled gardening tool with a flat, thin metal blade at a roughly 90 degree angle, used mainly for weeding and breaking up soil
Wheelbarrow: a hand-driven small cart with a single wheel at the front for carrying loads around the garden
Trowel: a small, handheld shovel
Shovel: a gardening tool with a long handle and a metal scoop, used for digging and transporting soil and other materials
Rake: a gardening tool with a long handle and short metal tines, used for spreading compost as well as turning and smoothing out soil
Cold Frame: a box shaped frame with four sides and a glass or plastic top, used to extend the growing season by allowing plants to be warmer than the surrounding area, as well as to protect plants
Gloves: gardening gloves are typically made from a canvas or thick cotton fabric with rubber dots on the palm and inside of fingers to provide protection, grip, and keep your hands clean from soil
I hope this will be a good point of reference for you as you get to know all the lingo of the gardening world. There were many times when I had to look up a term I wasn’t familiar with while reading one of the many gardening books that have taught me along the way. Enjoy this time of learning!
Activity
Vocabulary: Word Meaning, Word Web
Lesson Planet: Curated OER
Explore the multiple meanings of common homographs with this fun language arts activity. Given a series of word webs and a pile of definition cards, pupils complete each web by matching four different definitions to each target word.
15 mins
2nd — 3rd
English Language Arts
CCSS:
Adaptable
Activity
Vocabulary: Morphemic Elements, Root-O!
Lesson Planet: Curated OER
Young readers get to the root of unfamiliar vocabulary with a collaborative learning activity. Given a deck of root word cards and copies of a graphic organizer, pairs of students take turns flipping over cards and brainstorming…
30 mins
4th — 5th
English Language Arts
CCSS:
Adaptable
Worksheet
Plant Word Shapes (2)
Lesson Planet: Curated OER
In this parts of a plant worksheet, students look over 10 word shapes and then fill in each box with its appropriate plant part from the word box at the bottom of the worksheet.
K — 3rd
Career & Technical Education
Worksheet
The Honeycomb Challenge: Shapes and Colors
Lesson Planet: Curated OER
Play this game with young English language learners to help them learn color and shape vocabulary. As they make their way around the game board, they talk about the colors and shapes they pass and land on. Add math skills practice by…
2nd — 3rd
English Language Arts
Lesson Plan
Vocabulary Charts
Lesson Planet: Curated OER
Help your kids learn their vocabulary with a lesson plan and graphic organizer. After receiving a list of new vocabulary words, learners look up the definitions in dictionaries and fill in the worksheet. Additionally, they include a…
4th — 6th
English Language Arts
CCSS:
Adaptable
Activity
Vocabulary: Word Meaning, Word Clues
Lesson Planet: Curated OER
Young learners develop a deeper understanding of target vocabulary. In pairs, pupils independently complete a series of word clue cards, asking them to find information about key terms, including their definitions, synonyms, antonyms,…
30 mins
4th — 5th
English Language Arts
CCSS:
Designed
Worksheet
ESL Vocabulary Word Search: Shapes and Colors
Lesson Planet: Curated OER
Are your English language learners developing color-related vocabulary? Use this word search to reinforce their new vocabulary words! Some of the words are backwards and diagonal! Keep this in mind for young learners!
2nd — 4th
English Language Arts
Worksheet
Word Families: ake
Lesson Planet: Curated OER
Young learners create a book of words that use the letter combination ake. Six pages are included for them to cut and color, and there’s a space for them to trace the letters of the word as well as write the word independently. The…
1st — 3rd
English Language Arts
Interactive
Latin Roots Cern, Jur, Leg: Fill in the Blanks Quiz
Lesson Planet: Curated OER
MyVocabulary.com features three levels of words for each root; this fill-in-the-blanks quiz contains a word bank of beginner vocabulary containing the roots cern/cer/cre, jur/jus, and leg. You can print it out, or your learners can take…
4th — 8th
English Language Arts
Lesson Plan
Using Words as a Way into Rick Riordan’s The Lightning Thief
Lesson Planet: Curated OER
Use the Visual Thesaurus to predict the subject matter of Rick Riordan’s book The Lightning Thief. A pre-reading activity encourages middle schoolers to use context clues and word meaning to discover what the book is about. After they…
4th — 8th
English Language Arts
CCSS:
Adaptable
Pruning is defined, according to the dictionary, as “trimming (a tree, shrub, or bush) by cutting away dead or overgrown branches or stems, especially to increase fruitfulness and growth.”
Whether you’re just starting out with caring for plants, or you’re managing an indoor jungle, pruning plants is an activity that everyone can do at one point or another. It’s good for your plants for a variety of reasons: it helps keep pests and disease away, it prevents your plants from getting too sparse, and it allows your plants to retain a shape and size suitable for indoor spaces. In this journal post, we’ll cover everything you need to know to successfully trim and shape your plants, why it’s important, and different techniques to use for both vines and trees to encourage intentional growth.
The Basics
Successfully trimming and shaping your houseplants doesn’t require anything more than a good, clean pair of gardening shears or scissors and some general knowledge of how plants work.
Pruning and cutting away leaves, stems, and branches — in most cases — doesn’t harm your plant. In fact, it’s healthy to do this every now and then. Plants will benefit from a good trimming the most during spring and summer, which are their active growing seasons. Trimming can be done to both vines and trees to encourage new, fuller growth along the plants, as well as to get rid of any yellowing or dead sections. Whether you’re wanting to maintain a certain size, encourage branching, or achieve a certain look, pruning is one of the best ways to gain control over how your plant is growing.
Most houseplants can be pruned and cut, however, there are certain kinds that care should be taken with— these include most palms and tree ferns. Dead fronds and leaves can be removed, but these plants never form branches, so the top areas of growth shouldn’t be cut off, otherwise it will effectively kill the plant.
Pulling Away Yellow or Brown Leaves
Trimming or plucking away yellowing or dead leaves is an easy way to help prevent any unwelcome plant pests from settling onto your plant, which are attracted to decaying or dead leaves more than healthy ones, and they are more likely to appear on a struggling plant.
Pulling away yellowing or dead leaves is also a good way to keep your plant looking its best. When a leaf is yellowing, let the leaf fully turn yellow before pulling it off. When a leaf is on its way out, the leaf loses all of its chlorophyll (the molecules which make the leaf green), and the plant absorbs any leftover nutrients from the yellowing leaf. The leaf should be able to be pulled off easily without any kind of resistance. Any leaves that have turned brown and crispy can also be pulled off of a stem or branch without harming your plant.
Trimming off brown leaf edges will not harm the plant and is a way to make the overall plant look more appealing
Pro tip : try to trim in a way that mimics the original, natural shape of the leaf
Seasonal vs. Daily Pruning
Some tasks, when it comes to trimming and pruning houseplants, should be done during certain times of the year. It’s best to get any large amounts of pruning done during the spring or summer, when your plants are getting more sunlight and are actively growing. Trimming off a large amount of leaves, branches, or anything that will cut back a sizable amount of your plant, is best done during this time. In general, you don’t want to remove any more than 1/4 of the overall foliage of the plant. Hold back on any major pruning during the fall and winter — your plants won’t be growing as quickly, and it could take a longer time for them to put out new growth or recover from being trimmed too much. However, there are tasks that can easily be accomplished during any time of the year. Pulling off yellowing or brown leaves, trimming away a few stems or vines, or other smaller items, can all be done daily or as needed throughout the year to keep your plants looking healthy.
Pruning Trees
Like trees living outdoors, indoor trees should occasionally be pruned to help maintain their shape and growth. Likewise, if they’re getting too large for your space, it’s a good idea to shape and prune them. Pruning trees is also a great way to thin them out a bit. Not only does this make them more visually attractive, but it also improves air flow between leaves and branches, leading to an overall healthier plant.
Indoor trees, such as Ficus and Dracaenas, tend to grow vertically, but it is possible to encourage branching by trimming off the topmost point of growth. Doing this will force your plant to branch out from the sides of where you cut, rather than continuing to grow directly upwards. By branching off of this idea, you can control where and how your plant grows, allowing you to tailor your tree’s growth to suit your indoor space.
When an existing branch is pruned, new branches will sprout from beneath the area where the cut was made. In this photo, the Fiddle Leaf Fig tree is sprouting 3 new branches.
Here you can see where the main branch was pruned and two more branches have since grown and matured from below the cut.
Pruning Vines
Like trees, vines can be pruned regularly to keep them from getting too leggy and to encourage a fuller appearance. Vines such as Pothos and some varieties of Philodendrons benefit most from regular pruning. Besides pulling away dead or yellow leaves, it’s possible to get most vines to look bushier and more full via pruning. To accomplish this, trim either directly below a leaf or occasionally pinch off new growth with your fingers to get your plant to sprout new vining stems off of an old one — this keeps your plant looking compact and full, rather than having sparse, single vines trailing down a planter.
Propagating with Cuttings
You may be left wondering what to do with any branches, vines, or stems you’ve cut away from your plants. To preserve all parts of your plant, you can usually propagate them by sticking them directly into water or soil. Most branches root readily, as do cuttings from plants like Sansevieria, ZZ plants, Hoyas, and others. For vines such as Pothos, Philodendrons, and Monsteras, you’ll want to cut directly below a node or aerial root. Sticking this part into water or soil, will allow for water or soil roots to grow and sustain the cutting as it continues to grow.
Here, a Pothos cutting is beginning to root in water.
With all of this information, hopefully you’re well-equipped to prune and trim your plants to keep them looking (and being!) healthy and happy. If you have any questions, feel free to comment below or come visit us in one of our shops!
Written by: Egan Thorne
Photos by: Michelle Carr
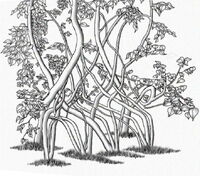
There are various methods of tree shaping.[1][2] There are strengths and weaknesses to each method as well commendable tree species for each process.[3] Some of these processes are still experimental,[4]: 154 [5] whereas others are still in the research stage.[6] These methods use a variety of horticultural and arboricultural techniques to achieve an intended design. Chairs, tables, living spaces and art may be shaped from growing trees. Some techniques used are unique to a particular practice, whereas other techniques are common to all, though the implementation may be for different reasons.[5] These methods usually start with an idea of the intended outcome. Some practitioners start with detailed drawings[7]: 7 or designs.[8] Other artists start with what the tree already has.[9]: 56–57 Each method has various levels of involvement from the tree shaper.[5][10]
Aeroponic cultureEdit
With Aeroponic culture, the roots of the tree are the main thing shaped by this method.[3] The oldest known living examples of woody plant shaping are the aeroponically cultured living root bridges built by the ancient War-Khasi people of the Cherrapunjee region in India. These are being maintained and further developed today by the people of that region. Aeroponic growing was first formally studied by W. Carter in 1942. Carter researched air culture growing and described «a method of growing plants in water vapor to facilitate examination of roots».[12][5] Later researchers, including L. J Klotz and G. G. Trowel, expanded on his work.[13] In 1957, F. W. Went described «the process of growing plants with air-suspended roots and applying a nutrient mist to the root section», and in it he coined the word ‘aeroponics’ to describe that process.
In 2008, root researcher and craftsman Ezekiel Golan described and applied for a patent for a process which allows the roots of some aeroponically grown woody plants to lengthen and thicken while still remaining flexible. At lengths of perhaps 6 metres (20 ft) or more, the soft roots can be formed into pre-determined shapes which will continue thickening after the shapes are formed and as they continue to grow.[2][3][11]
Researchers at Plantware Co with Tel Aviv University Professors Yoav Waisel and Amram Eshel realised that «soft roots»[6] when grown aeroponically, stay soft and malleable. Once the roots reach a suitable length to be molded and planted, the roots change and trigger lignification, hardening and thickening.[5][3] [14]
Newer techniques and applications, such as eco-architecture, may allow architects to design, grow, and form large permanent structures, such as homes, by shaping aeroponically grown plants and their roots.[6]
Instant tree shapingEdit
Instant tree shaping[2][15] is a widely used method.[3] It uses mature trees, perhaps 6–12 ft. (2–3.5 m) long[5][4]: 196 and 3-4in (7.6–10 cm) in trunk diameter.[5][4]: 172 An instantaneous form is created by bending, weaving and sometimes cutting or marking the trees into the desired shape. Then the shaping is held in place till the tree has grown enough annual rings to hold the shape, effectively casting it permanently into place.[5][16][17] Understanding a tree’s fluid dynamics is important to achieving the desired result.[1][9]: 69
Bending is used to achieve a design.[5][9] If the trees are bent at too sharp an angle it may break, which can be mostly avoided by un-localizing the pressure. This is achieved by making small bends or even cuts along the underside of the curve on the tree. The level of resistance depends on the speed of the tree’s growth.[5] Bends are then held in place for several years until their form is permanently cast.[5][9]: 80 The tree’s rate of growth determines the time necessary to overcome its resistance to the initial bending.[4]: 178 The work of bending and securing in this way might be accomplished in an hour or perhaps in an afternoon depending on the design.[15] Ring barking sometimes called girdling can be employed to help balance a design by slowing the growth of too-vigorous branches or stopping the growth of inopportunely placed branches, using different degrees of ring barking, from simple scoring to complete removal of a 3/8″-wide (1 cm) band of bark.[5][9]: 57, 69 This is a way to stop the tree from overgrowing a part or parts of the design, providing balance.[5] Creasing is folding trees such as willow and poplar over upon themselves, creating a right angle. This method is more radical than bending.[5][4]: 80
With this method it is possible to perform initial bending and grafting on a project in an hour, as with Peace in Cherry by Richard Reames,[4]: 193 [9]: 56–57 removing supports in as little as a year and following up with minimal pruning thereafter.[18] Though easy to do, the drawbacks to this method are many;[3] for example, the tree’s response to the efforts of the practitioner is difficult to forecast.[3]
In 1977 David Nash created the Ash Dome with 22 ash saplings, forming a 30ft ring in diameter. By cutting V-shaped notches into the trunk of the ash trees in the direction of the bending, the trees folded over into the bend. The dome is expected to have a 200 year lifespan.[5] In 1998 Marcel Kalberer and Sanfte Strukuren and their team built The Auerworld Palace in less than 1 month.[3] Weaving together living willows saplings to create the structural supports. Over time the entire palace is covered with the new growth of the willows.[5] In private gardens, public spaces and in schools more than 10,000 living willow constructions have been erected in Germany.[3]
Gradual tree shapingEdit
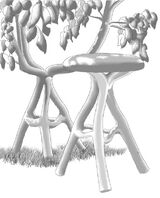
«Grownup furniture» by Chris Cattle
Gradual tree shaping as popularized by Pooktre starts with planning out[3] the designing and framing.[15][19][20][21] These are fundamental to the success of the piece.[20][21] The designing stage can take hours to complete.[8]
Once these are set up, young seedlings or saplings[7]: 4 [5] 3–12 in. (7.6–30.5 cm) long[20][21] are planted. The training starts with young seedlings, saplings or the stems of trees when they are very young,[7]: 4 which are gradually shaped while the tree is growing to form the desired shape.[22][3] There is a small area just behind the growing tip that forms the final shape.[19][23] The shaping zone,[19][23] it is the shaping of this area requires day to day or weekly guiding of the new growth.This is best done in the spring and summer months. [24] The growth is guided along predetermined design pathways; this may be a wooden jig[22][5] or complex wire design that can be accurate almost to the millimetere.[8] [5] To achieve the chosen shape a deep understanding of the tree’s growth patterns is necessary.[25]
With this method the time frame is longer than the other methods but predictable.[3] A chair design might take 8 to 10 years to reach maturity[26] [21] Some of Axel Erlandson’s trees took as long as 40 years to assume their finished shapes.[27] Given the amount of hands-on through out this process the designer has a large amount of control, which would give maximum potential with architecture.[5]
Common techniquesEdit
FramingEdit
Framing may be used for various purposes and might consist of any one or a combination of several materials, such as timber, steel, hollowed out trees,[28][5] complex wire designs,[8][5] wooden jigs,[22][5] or the tree itself, living[5][4]: 178 or dead.[29]: 58 It can be used in many project designs to support grafted joints until the grafts are well-established. Some processes might employ framing to hold a shape created by bending or fletching mature trees until the tissues have overcome their resistance to the initial bending and grown enough annual rings to cast the design permanently.[5][4] Others might use framing to support and shape the growth of young saplings[23][30] until they are strong enough to maintain an intended shape without support.[5] [23] Aeroponic roots can be held in place with frames to form desired shapes.[5]
An architecture example of framing that becomes part of the installation is Cathedral of Hornbeams. The growth of 80 young hornbeams trees is guided by the framing creating living columns. 1 meter in diameter and 12 meters high. The plan is once the wooden framing rots it becomes mulch for the living trees. Given the size of the trees the finished cathedral is expected to be 12 meters high, 80 meters by 8.7 meters.[5]
GraftingEdit
Grafting is a commonly employed technique that exploits the natural biological process of inosculation.[5] A branch or plant is cut and a piece of another plant is added and held in place. Various types of grafting all share the goal of encouraging the tissues of one plant to fuse with those of another.
Grafting is applied to create permanent connections and joints. In some cases, trees are grafted while they are growing,[31] while in other cases, mature trees may be intertwined and the stems of two or more trees are then grafted together to create chairs, ladders, and other fanciful sculptures.[32]
PruningEdit
Pruning can be used to balance a design by controlling and directing growth into a desired shape.[23][29]: 70 [30] Pruning above a leaf node can steer plant growth in the direction of the natural placement of that leaf bud.[5][4] Pruning may also be used to keep a design free of unwanted branches and to reduce canopy size.[23][30] Pruning is sometimes the only technique used to craft a project. Deciduous trees are mainly pruned in winter,[29]: 137 repeated over pruning of tree may stunt a tree or even kill it.[5]
TimingEdit
Using time as part of the construction is intrinsical to achieving this art form.[33]
DesigningEdit
The practitioners across this field have different approaches to how they design. From free form[9] to very detailed drawings[7]: 7 or designs.[8] Designs can incorporate inclusions, can be for either structural reasons or for aesthetic.[5] Some projects are for indoors and others outdoors or both. [6]
Tree shaping projects are usually designed with an idea of whether they are going to remain living or going to be a harvested piece.
- One approach is for the harvested piece to be grown outside and once harvested it will be put to use as an interior object.[34][2] A harvest piece will be dried and sometimes carved.[35]
- Ezekiel Golan and Yale Stav use Aeroponic culture for their Ficus roots to create interior shaped forms for indoor use which are to remain growing while inside the home.[34]
- Another design angle is to allow the trees of a project to mature in outside landscape and to remain living. Axel Erlandson and many others use this design style for their art.[34]
ToolsEdit
Bonsai tools can also be used
Various materials and tools may be used for creating, shaping, or molding a tree project. For example:- A wire frame can be created with fencing wire and tape[8] or a metal patio bench could be used as a design pattern. Lumber, pipe, rope, wire, string, yarn, twine, wire rope, rocks, sandbags, or other weighting objects, tape, and any number of other materials might be useful in effecting the design outcome. Some of the same tools that arborists, bonsai artists, gardeners, and other horticulturists use, are useful here as well, including hand pruners (secateurs), pruning knives, saws, and shovels for planting.[5] Shears and hedge trimmers are used less commonly, being perhaps better suited for establishment and foliage maintenance of topiary or sheared hedges.
Tree SpeciesEdit
Tree shapers when looking for a new tree species to try generally look for trees that grow well in the area, are less prone to insect damage, and are less susceptible to disease.[5] Given grafting and the trees ability of inosculation form a fundamental technique, trees that graft well are preferred in construction style projects. The region dictates which trees are primary of interest due to the geography and climate. The trees’ traits like lifespan and adaptability are useful to understand.[5] Any tree species has the potential for shaping. Each type of tree has its own quirks, but they can be understood with time and experience.[15]
Fullgrown, is currently working with ash, sycamore, hazel, sessile omarkak, red oak, crab apple and the common osier willow used for basketwork. Experimentation has shown that, surprisingly, oak in its early Test pieces to try out finished shaping Successful early grafting experiment of young shoots Growing timber for furniture growth can produce as quickly as the willow. That is, a chair in 4-5 years. Gavin finds the best contender is sycamore.
Some of the trees that have been shaped include:
Edit
- Bonsai – Japanese art of training plants to mimic miniature versions of large trees
- Topiary – Horticulture practice to shape trees and shrubs
- Espalier – Pruning/tying branches to flat structure
- Pleaching – Interwoven branches to form a hedge, fence or lattice
See alsoEdit
- Arthur Wiechula – Tree shaping theorist
- Christopher Cattle – British furniture designer and Tree shaping artist
- Richard Reames – American artist, arborsculptor, nurseryman, writer and public speaker
- Fab Tree Hab – Hypothetical Concept of ecological home design
- Gilroy Gardens – Family amusement and nature park
- Full Grown – Company that grows trees into furniture and sculpture
ReferencesEdit
- ^ a b
- ^ a b c d McKee, Kate (2012), «Living sculpture», Sustainable and water wise gardens, Westview: Universal Wellbeing PTY Limited, pp. 70–73
- ^ a b c d e f g h i j k l m Thomas Vallas; Luc Courard (25 May 2017). «Using nature in architecture Building a living house with mycelium and trees». Frontiers of Architectural Research. 6 (3): 318–328. doi:10.1016/j.foar.2017.05.003. ISSN 2095-2635.
- ^ a b c d e f g h i j Richard Reames (2005), Arborsculpture: Solutions for a Small Planet, Oregon: Arborsmith Studios, ISBN 0-9647280-8-7
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj Chithra, K.; Krishnan, K. Amritha (2015). Implementing Campus Greening Initiatives: Approaches, Methods and Perspectives (World Sustainability Series)Chapter Title:BIOTECTURE—A New Framework to Approach Buildings and Structures for Green Campus Design. Switzerland: Springer International Publishing. pp. 113–124. ISBN 978-3-319-11960-1.
- ^ a b c d «Eco-Architecture Could Produce «Grow Your Own» Homes». American Friends of Tel Aviv University. Archived from the original on 11 December 2009. Retrieved 6 May 2010.
- ^ a b c d e f g h i j k l m Erlandson, Wilma (2001), My father «talked to trees», Westview: Boulder, p. 22, ISBN 0-9708932-0-5
- ^ a b c d e f g h i j Volz, Martin (October–November 2008), «A Tree shaper’s life.» (PDF), Queensland Smart Farmer, archived from the original (PDF) on 23 July 2011
- ^ a b c d e f g Richard Reames; Delbol, Barbara (1995), How to Grow a Chair: The Art of Tree Trunk Topiary, ISBN 0-9647280-0-1
- ^ Title Turning young trees into living works of art Date 31 August 2014 Publisher Sunday Observer (Sri Lanka, India) HT Digital Streams Ltd.
- ^ a b US «A method of shaping a portion of a woody plant into a desired form is provided. The method is effected by providing a root of a woody plant, shaping the root into the desired form and culturing the root under conditions suitable for secondary thickening of the root.» 7328532, Golan, Ezekiel, «Method and a kit for shaping a portion of a woody plant into a desired form», issued 12 February 2008
- ^ Carter, W.A. (1942). A method of growing plants in water vapor to facilitate examination of roots. Phytopathology 732: 623-625.
- ^ Stoner, R.J. (1983). Rooting in Air. Greenhouse Grower Vol I No. 11.
- ^ Stav, Yael (4 July 2021). «Treenovations: a reliable, flexible tree-shaping method». INVIVO Progressive Sustainability. Archived from the original on 26 February 2021. Retrieved 4 July 2021.
- ^ a b c d e f Swati Balgi (September 2009), «Live Art» (PDF), Society Interiors Magazine, Prabhadevi, Mumbai: Magna Publishing
- ^ Rodkin, Dennis (25 February 1996), «The Gardener», Chicago Tribune Sunday
- ^ Oommen, Ansel (15 September 2013), «The Artful Science of Tree Shaping», www.permaculture.co.uk, archived from the original on 12 November 2013, retrieved 19 August 2021
- ^ a b c d e f g h i j k l m Link, Tracey (13 June 2008), «Senior project for Bachelor of Science degree in Landscape Architecture» (PDF), Arborsculpture: An Emerging Art Form and Solutions to our Environment, p. 41, archived from the original (PDF) on 25 February 2012
- ^ a b c Roger, Fox (December 2012), «Artist tree», Better Homes and Gardens Last, p. 140
- ^ a b c Cattle, Christopher. «How to grow your stool». Archived from the original on 18 March 2011. Retrieved 14 June 2010.
- ^ a b c d e f «Living Trees, Living Art». Archived from the original on 10 October 2011. Retrieved 8 May 2009.
- ^ a b c d e f g h Davies, David (1 June 1996). «Plant your own furniture. Watch it grow». The Independent. UK. Archived from the original on 6 June 2021. Retrieved 15 August 2011.
- ^ a b c d e f Peter Cook; Becky Northey (2012). Knowledge to Grow Shaped Trees. Australia: SharBrin Publishing Ptd Ltd. ISBN 978-1-921571-54-1.
- ^ a b c «Thinking like a tree». Good Woodworking. GW 296: 60–63. September 2015.
- ^ Title Flux Directed Branched Nanowire Growth via VLS-GLAD Author Allan Leo Beaudry A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Microsystems and Nanodevices Department of Electrical and Computer Engineering. University of Alberta Supervised by Dr. Michael Brett Year 2014.
- ^ a b c «Artists Shape Trees into Furniture and Art» (PDF), Farm Show Magazine, vol. 32, no. 4, p. 9, June–August 2008
- ^ Weston, Sarah (3 October 2006), Axel Erlandson’s Tree Circus, Mid-County Post
- ^ Yuka Yoneda. «Living Growing Root Bridges Are 100% Natural Architecture». Retrieved 12 April 2013.
- ^ a b c Hicks, Ivan; Rosenfeld, Richard; Whitworth, Jo (2007), Tricks with Trees, Pavilion Books, p. 160, ISBN 978-1-86205-734-0
- ^ a b c «Going on a ‘bender’«, The Queensland Times, p. 18, May 2012
- ^ Musitelui, Robin (30 May 1995), «Santa Cruz Sentinel Article», Architect rescue famous tree, p. 222
- ^ Ken Mudge; Jules Janick; Steven Scofield; Eliezer E. Goldschmidt (2009), Jules Janick (ed.), A History of Grafting (PDF), Issues in New Crops and New Uses, Purdue University Center for New Crops and Plants Products, orig. pub. John Wiley & Sons, Inc., pp. 442–443 Note large file: 8.04MB.
- ^ Zoë Hendon & Anne Massey (2019). Design, History and Time: New Temporalities in a Digital Age (1st ed.). Great Britain: Bloomsbury Publishing Plc. ISBN 978-1-350-06066-1.
- ^ a b c Smolina, O. O. (2019). «Variability of approaches to arborsculptures». Iop Conference Series: Materials Science and Engineering. IOP Publishing Ltd. 687 (5): 055035. Bibcode:2019MS&E..687e5035S. doi:10.1088/1757-899X/687/5/055035.
- ^ Edmistone, Leanne (25 August 2013). «Dare to be Different». Sunday Mail.
- ^ Mack, Daniel (31 December 1996), Making Rustic Furniture: The Tradition, Spirit, and Technique with Dozens of Project Ideas (Illustrated ed.), Lark Books, p. 160, ISBN 1-887374-12-4
- ^ «Only Natural Grown Chair». Shawano Leader Newspaper. Wisconsin Historical Society. 19 October 1922. Retrieved 15 May 2010.
- ^ a b c d e f Ladd, Dan (22 January 2009), Sculpturefest 2008: Daniel Ladd, retrieved 7 September 2021
- ^ Reddy, Jini (23 January 2010). «Trail of the Unexpected: The root masters of India». Cherrapunjee Holiday Resort. Retrieved 8 May 2010.
- ^ a b Mark Primack. «Pleaching». The NSW Good Wood Guide. Retrieved 10 May 2010.

Artist Peter Cook seated in a living garden chair grown via the Pooktre method
Tree shaping (also known as pooktre, arborsculpture, tree training, and by several other alternative names) is the practice of training living trees and other woody plants into artistic shapes and useful structures. There are a few different methods of achieving a shaped tree, which share a common heritage with other artistic horticultural and agricultural practices, such as pleaching, bonsai, espalier, and topiary, and employing some similar techniques. Most artists use grafting to deliberately induce the inosculation of living trunks, branches, and roots, into artistic designs or functional structures.
Tree shaping has been practiced for at least several hundred years, as demonstrated by the living root bridges built and maintained by the Khasi people people of India. Early 20th century practitioners and artisans included banker John Krubsack, Axel Erlandson with his famous circus trees, and landscape engineer Arthur Wiechula. Contemporary designers include «Pooktre» artists Peter Cook and Becky Northey, «arborsculpture» artist Richard Reames, and furniture designer Dr. Christopher Cattle, who grows «grownup furniture».
History[]
Living root bridges in Nongriat village, Meghalaya
Some species of trees exhibit a botanical phenomenon known as inosculation (or self-grafting); whether among parts of a single tree or between two or more individual specimens of the same (or very similar) species. Trees exhibiting this behavior are called inosculate trees.
The living root bridges of Cherrapunji, Laitkynsew, and Nongriat, in the present-day Meghalaya state of northeast India. These suspension bridges are handmade from the aerial roots of living banyan fig trees, such as the rubber tree. The pliable tree roots are gradually trained to grow across a gap, weaving in sticks, stones, and other inclusions, until they take root on the other side. There are specimens spanning over 100 feet, some can hold up to the weight of 50 people. The useful lifespan of the bridges, once complete, is thought to be 500–600 years. They are naturally self-renewing and self-strengthening as the component roots grow thicker.
Living trees were used to create garden houses in the Middle East, which later spread to Europe. In Cobham, Kent there are accounts of a three-story house that could hold 50 people.
Methods[]
There are various methods to achieving a shaped tree. These process use a variety of horticultural, arboricultural, and artistic techniques to craft an intended design. Chairs, tables, living spaces and art may be crafted from growing trees. Some techniques used for shaping trees are unique to a particular process, whereas other techniques are common to all, though the implementation may be for different reasons. These methods all start with an idea of the intended outcome. Some practitioners start with detailed drawings, or designs,< other artists start with what the tree already has. Each process has it own time frame and a different level of involvement from the tree trainer. Some of these processes are still experimental, whereas others are still in the research stage. The trees might then either remain growing, as with the living Pooktre garden chair, or perhaps be harvested as a finished work like John Krubsack’s chair.
Aeroponic culture[]
Treenovation created this chair using the techniques of Aeroponic root shaping.
The oldest known living examples of woody plant shaping are the aeroponically cultured living root bridges built by the ancient War-Khasi people of the Cherrapunjee region in India. These are being maintained and further developed today by the people of that region. Aeroponic growing was first formally studied by W. Carter in 1942, before the process had an English language name. Carter researched air culture growing and described «a method of growing plants in water vapor to facilitate examination of roots». Later researchers, including L. J Klotz and G. G. Trowel, expanded on his work. In 1957, F. W. Went described «the process of growing plants with air-suspended roots and applying a nutrient mist to the root section,» and in it he coined the word ‘aeroponics’ to describe that process. In 2008, root researcher and craftsman Ezekiel Golan described and secured a patent for a process which allows the roots of some aeroponically grown woody plants to lengthen and thicken while still remaining flexible. At lengths of perhaps 18 ft or more, the soft roots can be formed into pre-determined shapes which will continue thickening after the shapes are formed and as they continue to grow.
Newer techniques and applications, such as eco-architecture, may allow architects to design, grow, and form large permanent structures, such as homes, by shaping aeroponically grown plants and their roots.
Instant tree shaping[]
Arborsculpture bench by Richard Reames created using the techniques as described in his books How to grow a chair and Arborsculpture
Instant tree shaping starts with more mature trees, perhaps 6–12 ft. long and 3-4in in trunk diameter, which are woven into the desired design and held until cast. Understanding a tree’s fluid dynamics is important to achieving the desired result.
Bending is sometimes used to achieve a design. If a plant’s tissue is bent at too sharp an angle it may break, which can be mostly avoided by un-localizing the bend. This is achieved by making small bends along the curve of the tree. Bends are then held in place for several years until their form is permanently cast. The tree’s rate of growth determines the time necessary to overcome its resistance to the initial bending. The work of bending and securing in this way might be accomplished in an hour or perhaps in an afternoon depending on the design.
Ring barking is sometimes employed to help balance a design by slowing the growth of too-vigorous branches or stopping the growth of inopportunely placed branches, using different degrees of ring barking, from simple scoring to complete removal of a 3/8 in-wide band of bark.
Creasing is folding trees such as willow and poplar over upon themselves, creating a right angle. This method is more radical than bending.
With this method it is possible to perform initial bending and grafting on a project in an hour, as with Peace in Cherry by Richard Reames, removing supports in as little as a year and following up with minimal pruning thereafter.
Gradual tree shaping[]
«Grownup furniture» by Chris Cattle created using a gradual tree shaping method
Gradual tree shaping starts with designing and framing. These are fundamental to the success of the piece. Once these are set up, young seedlings or saplings 3–12 in. long are planted.
The training starts with young seedlings, saplings or the stems of trees when they are very young, and are gradually shaped while the tree is growing to form the desired shape. There is a small area just behind the growing tip that forms the final shape. The shaping zone, it is the shaping of this area requires day to day or weekly guiding of the new growth. The growth is guided along predetermined design pathways; this may be a wooden jig or complex wire design.
With this method the time frame is longer than the other methods. A chair design might take 8 to 10 years to reach maturity Some of Axel Erlandson trees’s took as long as 40 years to assume their finished shapes.
Common techniques[]
Grafting[]
Grafting is a common technique used by all the different methods. Grafting exploits the natural biological process of inosculation. Grafting is where a branch or plant is cut and a piece of another plant is added and held in place. There are various types of grafting, in all types the idea is to encourage the tissues of one plant to fuse with those of another.
Grafting is applied to create permanent connections and joins. In some cases the trees are grafted while they are growing in others the mature trees may be intertwining and then grafting together the stems of two or more trees in order to create chairs, ladders, and other fanciful sculptures.
Framing[]
Framing may be used for various purposes and might consist of any one or a combination of several materials, such as timber, steel, tubes made of hollow out trees, complex wire designs, or the tree itself living. It can be used in many project designs to support grafted joints until the grafts are well-established. Some process might employ framing to hold a shape created by bending or fletching mature trees until the tissues have overcome their resistance to the initial bending and grown enough annual rings to cast the design permanently. Others might use framing to support and shape the growth of young saplings until they are strong enough to maintain an intended shape without support.Still other approaches might employ frames to guide the roots of aeroponically grown trees into desired shapes.
Pruning[]
Pruning can be used to balance a design by controlling and directing growth into a desired shape. Pruning above a node can steer plant growth in the direction of the natural placement of that leaf bud. Pruning may also be used to keep a design free of unwanted branches and to reduce canopy size. Pruning is sometimes the only technique used to craft a project. Deciduous trees are mainly pruned in winter, while they are dormant above-ground, although sometimes it is necessary to prune them during the growing season. Trees repeatedly subjected to hard pruning may experience stunted growth, and some trees may not survive this treatment.
Pleaching[]
Pleaching is a technique used in the very old horticultural practice of hedge laying. Pleaching consists of first plashing living branches and twigs and then weaving them together to promote their inosculation. It is most commonly used to train trees into raised hedges, though other shapes are easily developed. Useful implementations include fences, lattices, roofs, and walls. Some of the outcomes of pleaching can be considered an early form of what is known today as tree shaping. In an early, labor-intensive, practical use of pleaching in medieval Europe, trees were installed in the ground in parallel hedgerow lines or quincunx patterns, then shaped by trimming to form a flat-plane grid above ground level. When the trees’ branches in this grid met those of neighboring trees, they were grafted together. Once the network of joints were of substantial size, planks were laid across the grid, upon which they built huts to live in, thus keeping the human settlement safe in times of annual flooding. Wooden dancing platforms were also built and the living tree branch grid bore the weight of the platform and dancers.
In late medieval European gardens through the 18th century, pleached allées, interwoven canopies of tree-lined garden avenues, were common.
Structure[]
Living grown structures have a number of structural mechanical advantages over those constructed of lumber and are more resistant to Leaf decomposition. While there are some decay organisms that can rot live wood from the outside, and though living trees can carry decayed and decaying heartwood inside them; in general, living trees decay from the inside out and dead wood decays from the outside in. Living wood tissue, particularly sapwood, wields a very potent defense against decay from either direction, known as CODIT. This protection applies to living trees only and varies among species.
Growing structures is not as easy as it would seem. Quick growing willows have been used to grow building structures, they provide support or protection. A young group of German architects are in the process of such a structure and they are continually monitored and checked. Once the trees are of age to be able to take on load-bearing weight they are tested for stability and strength by a structural engineer. Once this is approved the supporting framework is removed. Projects are limited to the trees’ weight loading ability and growth. This is being studied and the load capacity will be proved by testing on prototypes.
Design options[]
Becky’s Mirror by Pooktre
Designs may include abstract, symbolic, or functional elements. Some shapes crafted and grown are purely artistic; perhaps cubes, circles, or letters of an alphabet, while other designs might yield any of a wide variety of useful shapes, such as clothes hangers, laundry and wastepaper bins, ladders, furniture, tools, and tool handles. Eye-catching structures such as living fences and jungle gyms can also be grown, and even large architectural designs such as live archways, domes, gazebos, tunnels, and theoretically entire homes are possible with careful planning, planting, and culturing over time. The Human Ecology Design team (H.E.D.) at the Massachusetts Institute of Technology is designing homes that can be grown from native trees in a variety of climates.
Suitable trees are installed according to design specifications and then cultured over time into intended structures. Some designs may use only living, growing wood to form the structures, while others might also incorporate inclusions such as glass, mirror, steel and stone, any of which might be used either as either structural or aesthetic elements.Inclusions can be positioned in a project as it is grown and, depending on the design, may either be removed when no longer needed for support or left in place to become fixed inclusions in the growing tissue.
Chronology of notable practitioners[]
War-Khasi people[]
The ancient War-Khasi people of India worked with the aerial roots of native banyan fig trees, adapting them to create footbridges over watercourses. Modern people of the Cherrapunjee region carry on this traditional building craft. Roots selected for bridge spans are supported and guided in darkness as they are being formed, by threading long, thin, supple banyan roots through tubes made from hollowed-out trunks of woody grasses. Preferred species for the tubes are either bamboo or areca palm, or ‘kwai’ in Khasi language, which they cultivate for areca nuts. The Khasi incorporate aerial roots from overhanging trees to form support spans and safety handrails. Some bridges can carry fifty or more people at once. At least one example, over the Umshiang stream, is a double-decker bridge. They can take ten to fifteen years to become fully functional and are expected to last up to 600 years.
John Krubsack[]
John Krubsack was an American banker and farmer from Embarrass, Wisconsin. He shaped and grafted the first known grown chair, harvesting it in 1914. He lived from 1858 to 1941. He had studied tree grafting and become a skilled found-wood furniture crafter. The idea first came to him to grow his own chair during a weekend wood-hunting excursion with his son.
He started box elder seeds in 1903, selecting and planting either 28 or 32 of the saplings in a carefully designed pattern in the spring of 1907. In the spring of 1908, the trees had grown to six feet tall and he began training them along a trellis, grafting the branches at critical points to form the parts of his chair. In 1913, he cut all the trees except those forming the legs, which he left to grow and increase in diameter for another year, before harvesting and drying the chair in 1914; eleven years after he started the box elder seeds. Dubbed The Chair that Lived; it is the only known tree shaping that John Krubsack did. The chair is on permanent display in a plexiglas case at the entrance of Noritage Furniture; the furniture manufacturing business now owned by Krubsack’s descendants, Steve and Dennis Krubsack.
Axel Erlandson[]
Basket Tree by Axel Erlandson
Axel Erlandson was a Swedish American farmer who started training trees as a hobby on his farm in Hilmar, California, in 1925. He was inspired by observing a natural sycamore inosculation in his hedgerow. In 1945, he moved his family and the best of his trees from Hilmar to Scotts Valley, California and in 1947, opened an horticultural attraction called the Tree Circus.
Erlandson lived from 1884 to 1964; training more than 70 trees during his lifetime. He considered his methods trade secrets and when asked how he made his trees do this, he would only reply, «I talk to them.» His work appeared in the column of Ripley’s Believe It or Not! twelve times. 24 trees from his original garden have survived transplanting to their permanent home at Gilroy Gardens in Gilroy, California. His Telephone Booth Tree is on permanent display at the American Visionary Art Museum in Baltimore, Maryland and his Birch Loop tree is on permanent display at the Museum of Art and History in Santa Cruz, California. Both of these are preserved dead specimens.
Arthur Wiechula[]
19th century sketch by Arthur Wiechula of inosculated branches
Arthur Wiechula was a German landscape engineer who lived from 1868 to 1941. In 1926, he published Wachsende Häuser aus lebenden Bäumen entstehend (Developing Houses from Living Trees) in German. In it, he gave detailed illustrated descriptions of houses grown from trees and described simple building techniques involving guided grafting together of live branches; including a system of v-shaped lateral cuts used to bend and curve individual trunks and branches in the direction of a design, with reaction wood soon closing the wounds to hold the curves. He proposed growing wood so that it constituted walls during growth, thereby enabling the use of young wood for building. Weichula never built a living home, but he grew a 394′ wall of Canadian poplars to help keep the snow off of a section of train tracks.
Dan Ladd[]
Beginning stage of latticed arch
Dan Ladd is a Northampton, Massachusetts based American artist who works with trees and gourds. He began experimenting with glass, china, and metal inclusions in trees in 1977 in Vermont and started planting trees for Extreme Nature in 1978. He became inspired by inosculation he noticed in nature and by the growth of tree trunks around man-made objects such as fences and idle farm equipment. He shapes and grafts trees, including their fruits and their roots, into architectural and geometric forms. Ladd calls human-initiated inosculation ‘pleaching’ and calls his own work ‘tree sculpture’. Ladd binds a variety of objects to trees, for live wood to grow around and be incorporated, including teacups, bicycle wheels, headstones, steel spheres, water piping, and electrical conduit. He guides roots into shapes, such as stairs, using above-ground wooden and concrete forms and even shapes woody, hard-shelled Lagenaria gourds by allowing them to grow into detailed molds. A current project at the DeCordova and Dana Museum and Sculpture Park in Lincoln, Massachusetts incorporates eleven American Liberty Elm trees grafted next to each other to form a long hillside stair banister. Another of his installations, Three Arches, consists of three pairs of 14-foot sycamore trees, which he grafted into arches to frame different city views, at Frank Curto Park in Pittsburgh, Pennsylvania.
Nirandr Boonnetr[]
Nirandr Boonnetr is a Thai furniture designer and crafter. He became inspired as a child, both by a photograph of some unusually twisted coconut palms in southern Thailand and by a living fallen tree he noticed, which had grown new branches along its trunk, forming a kind of canopied bridge. His hobby began in 1980 because of his concern the Thailand forests are being ravaged by woodcarvers to the point that one day the industry would eventually carve itself out of existence. He began his first piece, a guava chair, around 1983. Originally intended as something for his children to climb and play on, the piece evolved into a living tree chair. In fifteen years he created six pieces of «living furniture,» including five chairs and a table. The Bangkok Post dubbed him the father of Living Furniture. Shortly thereafter, he presented a chair as a gift to her Royal Highness, Princess Sirindhorn. Nirandr Boonnetr has written a detailed, step-by-step booklet of instructions hoping his hobby of living furniture will spread to other countries. One of his chairs was exhibited in the Growing Village pavilion at the World’s Fair Expo 2005 in Nagakute, Aichi Prefecture, Japan.
Peter Cook and Becky Northey[]
People trees, by Pooktre
Peter Cook and Becky Northey are Australian artists who live in South East Queensland. Peter Cook became inspired to grow a chair in 1987, after visiting three figs trees in a remote corner of his property. He started the next day, with 7 willow cuttings. In 1988, he planted a wattle intended for harvest as a potted plant stand. Becky Northey moved to Peter’s property in 1995 and the two formed Pooktre.
Their methods involve guiding a tree’s growth along predetermined wired design pathways over a period of time. They shape growing trees both for living outdoor art and for intentional harvest. They most often use Myrobalan Plum for shaping. Examples of their functional artwork include a growing garden table, a harvested coffee table, hat stands, mirrors, and a gemstone neck piece.
Peter and Becky exhibited eight of their creations, including two people trees. in the Growing Village pavilion at the World’s Fair Expo 2005 in Nagakute, Aichi Prefecture, Japan. Their work was published in the annual book series, Ripley’s Believe It or Not.
Richard Reames[]
Richard Reames‘s Peace in Cherry
Richard Reames is an American nurseryman and author based in Williams, Oregon, where he owns and manages a nursery, and design studio collectively named Arborsmith Studios. He was inspired by the works of Axel Erlandson, and began sculpting trees in 1991 or 1992. He began his first experimental grown chairs in the spring of 1993.
In 1995, Reames wrote and published his first book, How to Grow a Chair: The Art of Tree Trunk Topiary. In it, he coined the word arborsculpture. In 2005, he published his second book, Arborsculpture: Solutions for a Small Planet. He has lectured in Australia and gives live demonstrations of bending and weaving a chair at garden shows, fairs and folk art festivals around America.
Christopher Cattle[]
Christopher Cattle’s grown stool in sycamore
Christopher Cattle is a retired furniture design professor from England. He started his first planting of furniture in 1996. According to Cattle, he developed an idea to train and graft trees to grow into shapes, which came to him in the late 1970s, in response to questions from students asking how to build furniture using less energy.Using various species of trees and wooden jigs to shape them, he has grown 15 three-legged stools to completion.
Cattle has multiple plantings in at least four different locations in England. He participates in woodland and craft shows in England and at the Big Tent at Falkland Palace in Scotland. He exhibited his grown stools at the World’s Fair Expo 2005 in the Growing Village pavilion at Nagakute, Japan.
He aims to encourage as many people as possible to grow their own furniture, and envisions that, «One day, furniture factories could be replaced by furniture orchards.» Cattle calls his works grown up furniture and grown stools, but also refers to them as grown furniture, calling them «the result of mature thinking.»
Mr. Wu[]
Mr. Wu is a Chinese pensioner who designs and crafts furniture in Shenyang, Liaoning, China. He has patented his technique of growing wooden chairs and as of 2005, had designed, grown, and harvested one chair, in 2004, and had six more growing in his garden. Wu uses young elm trees, which he says are pliant and do not break easily. He also says that it takes him about five years to grow a tree chair.
[]
Other artistic horticultural practices such as bonsai, espalier, and topiary share some elements and a common heritage, though a number of distinctions may be identified.
Bonsai[]
Bonsai is the art of growing trees in small containers. Bonsai uses techniques such as pruning, root reduction, and shaping branches and roots to produce small trees that mimic, full-sized mature trees. Bonsai is not intended for production of food, but instead mainly for contemplation by viewers, like most fine art.
Espalier[]
Espalier is the art and horticultural practice of training tree branches onto ornamental shapes along a frame for ascetic and fruit production by grafting, shaping and pruning the branches so that they grow flat, frequently in formal patterns, against a structure such as a wall, fence, or trellis. The practice is commonly used to accelerate and increase production in fruit-bearing trees and also to decorate flat exterior walls while conserving space.
Pleaching[]
Pleaching is a technique of weaving the branches of trees into a hedge commonly, deciduous trees are planted in lines, then pleached to form a flat plane on clear stems above the ground level. Branches are woven together and lightly tied. Branches in close contact may grow together, due to a natural phenomenon called inosculation, a natural graft. Pleach also means weaving of thin, whippy stems of trees to form a basketry affect.
Topiary[]
Topiary is the horticultural practice of shaping live trees, by clipping the foliage and twigs of trees and shrubs to develop and maintain clearly defined shapes, often geometric or fanciful. The hedge is a simple form of topiary used to create boundaries, walls or screens. Topiary always involves regular shearing and shaping of foliage to maintain the shape.
Plantings for the future[]
Fab Tree Hab 3D render
Three MIT designers Mitchell Joachim, Lara Greden and Javier Arbona created a concept of a living tree house which nourishes its inhabitants and merges within its environment. The project of Fab Tree Hab is expect to take a minimum of five years to grow the home. The plans are for the interior to by lined with clay and plastered to keep the weather outside and to look normal. The exterior is to be all-natural.
A Swedish architectural firm VisionDivision took part in a week-long workshop at the Italian university Politecnico di Milano. with the students. The result was an 80 year plan of a living cheery tree dome in an hour glass shape and grown furniture. Framing for the dome, table and a lawn chair were build. 10 Japanese cherry trees were planted in a diameter of eight meter circle. Four of these trees are to be living staircases to a future top level. The stair trees will have their branches grafted into each other to form the rungs. VisionDivision’s architects helped the students and instructors to create an easy maintenance plan for future gardeners of the university.
Ferdinand Ludwig designed this tower as part of his doctoral thesis with the help of Prof. Dr. Speck. «Speck become the botanical co-supervisor» said Ferdinand. Growing at the University of Stuttgart is a three storey tower of living white willows (Salix alba). This nine meter tall construction is almost fully grown, with a base area of around eight square meters.
The framing of made up of mainly steel scaffolding which is supporting the growing trees, while keeping them to the correct form. They started with 400 white willow (Salix alba) grown in baskets on muiltable levels with one row of willows planted into the ground. Once the trees where two meters tall they were planted at the different levels of the tower. These plants are then trained to the design.
The root system of the bottom level of willows needs to develop large enough to support the willows on the above levels. So that the scaffold becomes obsolete and then it and the watering and ferilizing baskets can be removed altogether.
The trees are grafted together with the objective of all the different plants eventually become a single organism. The overall aim is to have s living structure with the strength support itself and to carry a working load. Ferdinand predicts the tower will stable enough to support itself in five-ten years. Ferdinand does state » However, these are only estimates.»
Alternative names[]
The practice of shaping living trees has several names. Practitioners may have their own name for their techniques, so a standard name for the practice has not emerged. Richard Reames calls the practice «arborsculpture»; Dan Ladd calls his work «tree sculpture»; Nirandr Boonnetr’s work is called «living furniture»; Christopher Cattle calls his works «grown up furniture» and «grown stools»; while Peter Cook and Becky Northey call their work «Pooktre».
The following names are also encountered:
- Arbortecture
- Biotecture/Biotechture
- Grown furniture
- Living Art
- Pleaching
- Tree training
In fiction and art[]
In 1516, Jean Perréal painted an allegorical image, La complainte de nature à l’alchimiste errant, (The Lament of Nature to the Wandering Alchemist), in which a winged figure with arms crossed, representing nature, sits on a tree stump with a fire burning in its base, conversing with an alchemist in an ankle-length coat, standing outside of his stone-laid shoreline laboratory. Live resprouting shoots emerge from either side of the tree stump seat to form a fancifully twined and inosculated two-story-tall chair back.
In 1758, Swedish scientist, philosopher, Christian mystic, and theologian Emanuel Swedenborg published Earths in the Universe, in which he wrote of visiting another planet where the residents dwelled in living groves of trees, whose growth they had planned and directed from a very young stage into living quarters and sanctuaries.
In the late 19th century, Styrian Christian mystic and visionary Jakob Lorber published The Household of God. In it, he wrote about the wisdom of planting trees in a circle, because once grown together, the ring of trees would be a much better house than could be built.
For the practice of shaping trees and shrubs by clipping the foliage, see Topiary.
A chair formed by tree shaping[1]
Tree shaping (also known by several other alternative names) uses living trees and other woody plants as the medium to create structures and art. There are a few different methods[2] used by the various artists to shape their trees, which share a common heritage with other artistic horticultural and agricultural practices, such as pleaching, bonsai, espalier, and topiary, and employing some similar techniques. Most artists use grafting to deliberately induce the inosculation of living trunks, branches, and roots, into artistic designs or functional structures.
Tree shaping has been practiced for at least several hundred years, as demonstrated by the living root bridges built and maintained by the Khasi people of India. Early 20th century practitioners and artisans included banker John Krubsack, Axel Erlandson with his famous circus trees, and landscape engineer Arthur Wiechula. Several contemporary designers also produce tree shaping projects.
History[edit]
Some species of trees exhibit a botanical phenomenon known as inosculation (or self-grafting); whether among parts of a single tree or between two or more individual specimens of the same (or very similar) species. Trees exhibiting this behavior are called inosculate trees.[3]
The living root bridges of Cherrapunji, Laitkynsew, and Nongriat, in the present-day Meghalaya state of northeast India are examples of tree shaping.[4] These suspension bridges are handmade from the aerial roots of living banyan fig trees, such as the rubber tree.[5] The pliable tree roots are gradually shaped to grow across a gap, weaving in sticks, stones, and other inclusions, until they take root on the other side.[5] This process can take up to fifteen years to complete.[6] There are specimens spanning over 100 feet, some can hold up to the weight of 50 people.[7][8] The useful lifespan of the bridges, once complete, is thought to be 500–600 years. They are naturally self-renewing and self-strengthening as the component roots grow thicker.[8]
Living trees were used to create garden houses in the Middle East, a practice which later spread to Europe. In Cobham, Kent there are accounts of a three-story house that could hold 50 people.[9][4]
Pleaching is a technique used in the very old horticultural practice of hedge laying. Pleaching consists of first plashing living branches and twigs and then weaving them together to promote their inosculation. It is most commonly used to train trees into raised hedges, though other shapes are easily developed. Useful implementations include fences, lattices, roofs, and walls.[3][10] Some of the outcomes of pleaching can be considered an early form of what is known today as tree shaping.[citation needed] In an early, labor-intensive, practical use of pleaching in medieval Europe, trees were installed in the ground in parallel hedgerow lines or quincunx patterns, then shaped by trimming to form a flat-plane grid above ground level. When the trees’ branches in this grid met those of neighboring trees, they were grafted together. Once the network of joints were of substantial size, builders laid planks across the grid, upon which they built huts to live in, thus keeping the human settlement safe in times of annual flooding.[3] Wooden dancing platforms were also built and the living tree branch grid bore the weight of the platform and dancers.[11]
In late medieval European gardens through the 18th century, pleached allées, interwoven canopies of tree-lined garden avenues, were common.[citation needed]
Methods[edit]
«Grownup furniture» by Chris Cattle
There are a few different methods [2] of shaping trees. There is aeroponic culture, instant tree shaping [13] and gradual tree shaping.[13]
Aeroponic culture uses aeroponics, a process of growing tree roots in a nutrient rich mist. Once the roots are of a desired length for the pre-determined design they are shaped as they are planted.[14][12] This technique may be used in part to help form large permanent structures, such as eco-architecture.[15]
The oldest known root shaping are the living root bridges built by the ancient War-Khasi people of the Cherrapunjee region in India.
Instant tree shaping [14][13] uses trees 2 to 3 m (6.6 to 9.8 ft).[16]: 196 The trees are bent and woven into different designs and held until cast.[17][18] Bends are then held in place for several years until their form is permanently cast.[19]: 80 With this method it is possible to perform initial bending and grafting on a project in an hour, as with Peace in Cherry by Richard Reames,[16]: 193 [19]: 56–57
Some techniques of this method are bending,[19] and un-localizing the bend. Ring barking is sometimes employed to help balance a design.[19]: 57, 69 Creasing is folding trees such as willow and poplar over upon themselves.[16]: 80
Gradual tree shaping [13][20] starts with designing and framing.[20][21] Young seedlings or saplings[22]: 4 3–12 in. (7.6–30.5 cm) long[23][21] are planted. The growth is guided along predetermined design pathways; this may be a wooden jig [9] or a complex wire design.[24] The shaping zone is a small area just behind the growing tip that forms the final shape.[20]
[25] This zone requires day to day or weekly guiding of the new growth. To achieve a finished piece takes longer with this method. A chair design might take 8 to 10 years to reach maturity.[26] Some of Axel Erlandson’s trees took 40 years to assume their finished shapes.[27]
Common techniques[edit]
Some techniques are common to all the above methods though sometimes they are used differently for each.
Framing might consist of a combination or any one of several materials, including the tree itself, living [16]: 178 or dead.[28]: 58
Grafting is a commonly employed technique that exploits the natural biological process of inosculation. A branch is cut and held in place, it can be of the same plant or another cultivar of the plant. Grafting is applied to create permanent connections and joints.
Pruning can be used to balance a design by controlling and directing growth into a desired shape.[25][28]: 70 [29]
Timing is used as part of the construction is intrinsic to achieving this art form.[clarification needed][30][failed verification]
Structure[edit]
Living grown structures have a number of structural mechanical advantages over those constructed of lumber[citation needed] and are more resistant to decay. While there are some decay organisms that can rot live wood from the outside, and though living trees can carry decayed and decaying heartwood inside them; in general, living trees decay from the inside out and dead wood decays from the outside in.[31] Living wood tissue, particularly sapwood, wields a very potent defense against decay from either direction, known as compartmentalization. This protection applies to living trees only and varies among species.
Growing structures is not as easy as it would seem.[32] Quick growing willows have been used to grow building structures, they provide support or protection.[32] A young group of German architects are in the process of such a structure and they are continually monitored and checked.[32] Once the trees are of age to be able to take on load-bearing weight they are tested for stability and strength by a structural engineer.[32] Once this is approved the supporting framework is removed.[32] Projects are limited to the trees’ weight loading ability and growth.[32] This is being studied and the load capacity will be proved by testing on prototypes.[33]
Design options[edit]
Designs may include abstract, symbolic, or functional elements. Some shapes crafted and grown are purely artistic; perhaps cubes, circles, or letters of an alphabet, while other designs might yield any of a wide variety of useful shapes, such as clothes hangers,[34] laundry and wastepaper bins,[34] ladders,[35] furniture,[36] tools, and tool handles. Eye-catching structures such as living fences and jungle gyms[35] can also be grown, and even large architectural designs such as live archways, domes,[36] gazebos,[35] tunnels, and theoretically entire homes[15] are possible with careful planning, planting, and culturing over time.[11] The Human Ecology Design team (H.E.D.) at the Massachusetts Institute of Technology is designing homes that can be grown from native trees in a variety of climates.[37]
Suitable trees are installed according to design specifications and then cultured over time into intended structures. Some designs may use only living, growing wood to form the structures, while others might also incorporate inclusions [13][25] such as glass, mirror, steel and stone, any of which might be used either as either structural or aesthetic elements.[25] Inclusions can be positioned in a project as it is grown and, depending on the design, may either be removed when no longer needed for support or left in place to become fixed inclusions in the growing tissue.[28]: 117
The befit of using trees to grow a design which is then harvested for furniture, is that these pieces are stronger than the results of conventional manufacturing process. As the grain of the timber flows through the design instead of being chopped into smaller pieces then glued back together to form the design. All the joins of a shaped tree are grafted forming a stronger bond than a manufactured piece.[9]
Environmental benefits[edit]
Shaped tree projects can play a role in mitigating the imbalance of carbon dioxide-oxygen that happens in cities, creating a microclimate that could be soothing to human habitation. The types of projects that could work in this environment would be playground equipment, road furniture, walkways with over-bridges and bus shelters. This increased growth of trees would improve the shade and create a fresh wind channel. When choosing the trees to use a fruit tree would have the added use of giving food as well. It can be renewable in the long run and when they die they can be used as fertilizer.[38]
The trees and shaped roots can hold the soil preventing soil erosion and forestalling landslides.[39] In the right circumstances the trees could be planted over landfills and garbage dumps. Biodegradable waste could be used to help the trees remain healthily.[38]
Chronology of notable practitioners[edit]
War-Khasi people[edit]
The ancient War-Khasi people of India worked with the aerial roots of native banyan fig trees, adapting them to create footbridges over watercourses. Modern people of the Cherrapunjee region carry on this traditional building craft. Roots selected for bridge spans are supported and guided in darkness as they are being formed, by threading long, thin, supple banyan roots through tubes made from hollowed-out trunks of woody grasses. Preferred species for the tubes are either bamboo or areca palm, or ‘kwai’ in Khasi, which they cultivate for areca nuts. The Khasi incorporate aerial roots from overhanging trees to form support spans and safety handrails. Some bridges can carry fifty or more people at once. At least one example, over the Umshiang stream, is a double-decker bridge. They can take ten to fifteen years to become fully functional and are expected to last up to 600 years.[citation needed]
John Krubsack[edit]
John Krubsack was an American banker and farmer from Embarrass, Wisconsin. He shaped and grafted the first known grown chair,[40] harvesting it in 1914. He lived from 1858 to 1941. He had studied tree grafting and become a skilled found-wood furniture crafter.[41] The idea first came to him to grow his own chair during a weekend wood-hunting excursion with his son.
He started box elder seeds in 1903, selecting and planting either 28[41] or 32[42] of the saplings in a carefully designed pattern in the spring of 1907.[41] In the spring of 1908, the trees had grown to six feet tall and he began training them along a trellis, grafting the branches at critical points to form the parts of his chair.[41] In 1913, he cut all the trees except those forming the legs, which he left to grow and increase in diameter for another year, before harvesting and drying the chair in 1914; eleven years after he started the box elder seeds.[41] Dubbed The Chair that Lived; it is the only known tree shaping that John Krubsack did.[41][42] The chair went on tour via several exhibitions around the US and was featured in Ripley’s Believe It or Not!.[41] The chair is on permanent display in a Plexiglas case at the entrance of Noritage Furniture; the furniture manufacturing business now owned by Krubsack’s descendants, Steve and Dennis Krubsack.[16]
Axel Erlandson[edit]
Axel Erlandson was a Swedish American farmer who started training trees as a hobby on his farm in Hilmar, California, in 1925. He was inspired by observing a natural sycamore inosculation in his hedgerow.[3] In 1945, he moved his family and the best of his trees from Hilmar to Scotts Valley, California, and in 1947,[16] opened an horticultural attraction called the Tree Circus.
Erlandson lived from 1884 to 1964; training more than 70 trees during his lifetime. He considered his methods trade secrets and when asked how he made his trees do this, he would only reply, «I talk to them.»[22] His work appeared in the column of Ripley’s Believe It or Not! twelve times.[43] 24 trees from his original garden have survived transplanting to their permanent home at Gilroy Gardens in Gilroy, California. His Telephone Booth Tree is on permanent display at the American Visionary Art Museum in Baltimore, Maryland[37] and his Birch Loop tree is on permanent display at the Museum of Art and History in Santa Cruz, California. Both of these are preserved dead specimens.
Arthur Wiechula[edit]
19th-century sketch by Arthur Wiechula of inosculated branches
Arthur Wiechula was a German landscape engineer who lived from 1868 to 1941. In 1926, he published Wachsende Häuser aus lebenden Bäumen entstehend (Developing Houses from Living Trees) in German.[44][45] In it, he gave detailed illustrated descriptions of houses grown from trees and described simple building techniques involving guided grafting together of live branches; including a system of v-shaped lateral cuts used to bend and curve individual trunks and branches in the direction of a design, with reaction wood soon closing the wounds to hold the curves.[46] He proposed growing wood so that it constituted walls during growth, thereby enabling the use of young wood for building.[46] Weichula never built a living home, but he grew a 394′ wall of Canadian poplars to help keep the snow off of a section of train tracks.[44]
Dan Ladd[edit]
Dan Ladd is a Northampton, Massachusetts based American artist who works with trees and gourds. He began experimenting with glass, china, and metal inclusions in trees in 1977 in Vermont and started planting trees for Extreme Nature in 1978.[47] He became inspired by inosculation he noticed in nature and by the growth of tree trunks around man-made objects such as fences and idle farm equipment.[47] He shapes and grafts trees, including their fruits and their roots, into architectural and geometric forms.[47] Ladd calls human-initiated inosculation ‘pleaching’ and calls his own work ‘tree sculpture’.[47] Ladd binds a variety of objects to trees, for live wood to grow around and be incorporated, including teacups, bicycle wheels, headstones, steel spheres, water piping, and electrical conduit.[47] He guides roots into shapes, such as stairs, using above-ground wooden and concrete forms and even shapes woody, hard-shelled Lagenaria gourds by allowing them to grow into detailed molds.[48] A current project at the DeCordova and Dana Museum and Sculpture Park in Lincoln, Massachusetts incorporates eleven American Liberty Elm trees grafted next to each other to form a long hillside stair banister. Another of his installations, Three Arches, consists of three pairs of 14-foot sycamore trees, which he grafted into arches to frame different city views, at Frank Curto Park in Pittsburgh.[37][49]
Nirandr Boonnetr[edit]
Nirandr Boonnetr is a Thai furniture designer and crafter. He became inspired as a child, both by a photograph of some unusually twisted coconut palms in southern Thailand and by a living fallen tree he noticed, which had grown new branches along its trunk, forming a kind of canopied bridge.[16] His hobby began in 1980 because of his concern the Thailand forests are being ravaged by woodcarvers to the point that one day the industry would eventually carve itself out of existence.[50] He began his first piece, a guava chair, c. 1983.[16] Originally intended as something for his children to climb and play on, the piece evolved into a living tree chair.[16]: 91 In fifteen years he created six pieces of «living furniture»,[50] including five chairs and a table. The Bangkok Post dubbed him the father of Living Furniture.[16][51] Shortly thereafter, he presented a chair as a gift to her Royal Highness, Princess Sirindhorn. Nirandr Boonnetr has written a detailed, step-by-step booklet of instructions hoping his hobby of living furniture will spread to other countries.[50] One of his chairs was exhibited in the Growing Village pavilion at the World’s Fair Expo 2005 in Nagakute, Aichi, Japan.
Peter Cook and Becky Northey[edit]
Peter Cook and Becky Northey of Pooktre are Australian artists who live in South East Queensland. Cook began to grow his first chair in 1987 with 7 willow cuttings.[52] He was inspired by three fig trees on his property.[53][52] They were the featured artists at the Growing Village pavilion showing 8 pieces of grown art at the World’s Expo 2005 in Nagakute, Aichi Prefecture, Japan.[54]
Their methods involve guiding the tree’s growth along predetermined wire design pathways over a period of time.[14][26] They shape growing trees both for living outdoor art and for intentional harvest. They most often use Myrobalan Plum for shaping.[24]
Richard Reames[edit]
Richard Reames is an American nurseryman and author based in Williams, Oregon, where he owns and manages a nursery, and design studio collectively named Arborsmith Studios.[55] He was inspired by the works of Axel Erlandson,[16]: 150 [19]: 16 [56] and began sculpting trees in 1991[57] or 1992.[28] He began his first experimental grown chairs [19]: 57 in the spring of 1993.[19]: 85
In 1995, Reames wrote and published his first book, How to Grow a Chair: The Art of Tree Trunk Topiary. In it, he coined the word arborsculpture.[19] His second book, Arborsculpture: Solutions for a Small Planet was published in 2005.[16]
Christopher Cattle[edit]
Christopher Cattle’s grown stool in sycamore
Christopher Cattle is a retired furniture design professor from Oxford England.[58] He started his first planting of furniture in 1996.[9] According to Cattle, in the late 1970s he developed an idea to train and graft trees to grow into shapes[59] in response to questions from students asking how to build furniture using less energy.[58] Using various species of trees and wooden jigs to shape them,[23] he has grown 15 three-legged stools to completion.[citation needed]
He hopes to inspire others to grow their own furniture,[37][59] and envisions that, «One day, furniture factories could be replaced by furniture orchards.»[37] He calls his works «grown up furniture», «grown stools»,[58][60] and «grown furniture», calling them «the result of mature thinking.»[58]
Mr. Wu[edit]
Mr. Wu is a Chinese pensioner who designs, crafts and grows furniture in Shenyang, Liaoning, China. He’s been practicing this from 2000.[61]
[62][63] He enjoys some worldwide fame.[64] He has patented his technique of growing wooden chairs and as of 2005, had designed, grown, and harvested one chair, in 2004. He had six more growing in his garden.[63] Wu uses young elm trees,[65] which he says are pliant and do not break easily.[63] He also says that it takes him about five years to grow a tree chair.[62] He now uses his finished chairs within his home. With the hope of inspiring others to grow furniture.[61]
Gavin Munro[edit]
Gavin Munro is a designer who grows chairs, lamps, mirror frames and tables[66][67] by training trees in his chair orchard located at Wirksworth, in Derbyshire, England.[68] Munro co-founded Full Grown in 2005.
[edit]
Other artistic horticultural practices such as bonsai, espalier, and topiary share some elements and a common heritage, though a number of distinctions may be identified.
Bonsai[edit]
Bonsai is the art of growing trees in small containers. Bonsai uses techniques such as pruning, root reduction, and shaping branches and roots to produce small trees that mimic full-sized mature trees. Bonsai is not intended for production of food, but instead mainly for contemplation by viewers, like most fine art.[69][70]
Espalier[edit]
Espalier is the art and horticultural practice of training tree branches onto ornamental shapes along a frame for aesthetic and fruit production by grafting, shaping and pruning the branches so that they grow flat, frequently in formal patterns, against a structure such as a wall, fence, or trellis.[71] The practice is commonly used to accelerate and increase production in fruit-bearing trees and also to decorate flat exterior walls while conserving space.[71]
Pleaching[edit]
Pleaching is a technique of weaving the branches of trees into a hedge commonly, deciduous trees are planted in lines, then pleached to form a flat plane on clear stems above the ground level. Branches are woven together and lightly tied.[72] Branches in close contact may grow together, due to a natural phenomenon called inosculation, a natural graft. Pleach also means weaving of thin, whippy stems of trees to form a basketry affect.[73]
Topiary[edit]
Topiary is the horticultural practice of shaping live trees, by clipping the foliage and twigs of trees and shrubs to develop and maintain clearly defined shapes,[74] often geometric or fanciful. The hedge is a simple form of topiary used to create boundaries, walls or screens. Topiary always involves regular shearing and shaping of foliage to maintain the shape.
Plantings for the future[edit]
The Fab Tree Hab[edit]
Three MIT designers – Mitchell Joachim, Lara Greden and Javier Arbona – created a concept of a living tree house which nourishes its inhabitants and merges with its environment.[38][75] The project of Fab Tree Hab is expected to take a minimum of five years to grow the home.[76] The plans are for the interior to be lined with clay and plastered to keep the weather outside and to look normal. The exterior is to be all natural.[76]
The Patient Gardener[edit]
A Swedish architectural firm VisionDivision took part in a week-long workshop at the Italian university Politecnico di Milano[1] with the students. The result was an 80-year plan [77] of a living cherry tree dome in an hourglass shape and grown furniture. Nov 8 2011,10 Japanese cherry trees were planted with the Framing for the dome, table and a lawn chair were built. Ten Japanese cherry trees were planted in a diameter of eight-meter circle. Four of these trees are to be living staircases to a future top level. The stair trees will have their branches grafted into each other to form the rungs.[1][77] VisionDivision’s architects helped the students and instructors to create an easy maintenance plan for future gardeners of the university.[77]
Baubotanik Tower[edit]
Ferdinand Ludwig designed this tower as part of his doctoral thesis with the help of Prof. Dr. Speck. «Speck become the botanical co-supervisor» said Ferdinand. Growing at the University of Stuttgart is a three-storey tower of living white willows (Salix alba). This nine-meter-tall construction is almost fully grown, with a base area of around eight square meters.[11]
[33] : 86
The framing is made up of mainly steel scaffolding which is supporting the growing trees, while keeping them to the correct form. They started with 400 white willow (Salix alba) grown in baskets on multiple levels with one row of willows planted into the ground. Once the trees were two meters tall, they were planted at the different levels of the tower. These plants are then trained to the design.[11][33]
The root system of the bottom level of willows needs to develop large enough to support the willows on the above levels, so that the scaffold becomes obsolete and then it and the watering and fertilising baskets can be removed altogether.[33] : 86
The trees are grafted together with the objective of all the different plants eventually becoming a single organism. The overall aim is to have a living structure with the strength to support itself and to carry a working load. Ferdinand predicts the tower will be stable enough to support itself in five to ten years.[33] Ferdinand does state «However, these are only estimates.»[11]
Assessment[edit]
The advantages are trees can improve the habitation by generating more oxygen, giving shade and reuse of waste water creating a micro climate. Living trees are less prone to rot than timber via a process called compartmentalization. The joins are stronger than man made joinery. Mostly resistant to earthquakes and tsunamis.[38]
Some issues are the lack of working knowledge of how trees grow by architects and others. The speed of growth is unpredictable and they can grow in unwanted ways — thus creating a need to make plans adjustable. Trees can only reach a specific height and size dictated by their species. The environment can have a large impact on the growth and health of the trees.[38]
Alternative names[edit]
The practice of shaping living trees has several names. Practitioners may have their own name for their techniques, so a standard name for the various practices has not emerged.[54] «Arborsculpture»,[57][78][79] «tree sculpture»,[47] «living furniture»,[51] and other names have been used.[58][80][81]
The following names are also encountered:
- Arbortecture[57][78]
- Biotecture/Biotechture[10][82][38]
- Grown furniture[9][10][54]
- Living Art[13][37][83]
- Pleaching[3][44][84]
- Tree training[44][28][85]
- Baubotanik[86]
In fiction and art[edit]
In 1516, Jean Perréal painted an allegorical image,[57] La complainte de nature à l’alchimiste errant, (The Lament of Nature to the Wandering Alchemist), in which a winged figure with arms crossed, representing nature, sits on a tree stump with a fire burning in its base, conversing with an alchemist in an ankle-length coat, standing outside of his stone-laid shoreline laboratory. Live resprouting shoots emerge from either side of the tree stump seat to form a fancifully twined and inosculated two-story-tall chair back.[87][88][89]
In 1758, Swedish scientist, philosopher, Christian mystic, and theologian Emanuel Swedenborg published Earths in the Universe, in which he wrote of visiting another planet where the residents dwelled in living groves of trees, whose growth they had planned and directed from a very young stage into living quarters and sanctuaries.[78][90]
In the late 19th century, Styrian Christian mystic and visionary Jakob Lorber published The Household of God. In it, he wrote about the wisdom of planting trees in a circle, because once grown together, the ring of trees would be a much better house than could be built.[78][91]
In J. R. R. Tolkien’s popular fiction, The Lord of the Rings, elves were able to shape trees by singing,[92] and in Lothlórien, a forest described therein, trees were shaped into homes and walkways.
There are also tree-shaping elves in the 1978 comic book series Elfquest. They created homes, bows, animal forms, and other things to grow instantly from living trees. Most notable of these elves are Redlance and Goodtree.
See also[edit]
- Land art
- Landscaping
References[edit]
- ^ a b c Fionnuala Fallon (3 March 2012). «The trees that shape our lives». The Irish Times. Ireland.
- ^ a b
- ^ a b c d e Mark Primack. «Pleaching». The NSW Good Wood Guide. Archived from the original on 30 September 2009. Retrieved 10 May 2010.
- ^ a b Title Turning young trees into living works of art Date 31 August 2014 Publisher Sunday Observer (Sri Lanka, India) HT Digital Streams Ltd.
- ^ a b Lewin, Brent (2012), «November Volume 2012 Article», India’s living Bridges, Reader’s Digest Australia, pp. 82–89, EAN 9311484018704, archived from the original on 6 June 2013
- ^ Baker, Russ (6 October 2011). «Re-envisioning our environment». Business Insider. Archived from the original on 5 November 2011. Retrieved 9 June 2021.
- ^ «Living Growing Root Bridges Are 100% Natural Architecture». 11 August 2009. Archived from the original on 21 May 2013. Retrieved 12 April 2013.
- ^ a b «Living Root Bridge«. Online Highways LLC. 21 October 2005. Archived from the original on 4 August 2018. Retrieved 7 May 2010.
- ^ a b c d e David Davies (1 June 1996). «Plant your own furniture. Watch it grow». The Independent. UK. Archived from the original on 8 November 2012. Retrieved 15 August 2011.
- ^ a b c Fischbacher, Thomas (2007), Botanical Engineering (PDF), School of Engineering Sciences @ University of Southampton, archived from the original (PDF) on 22 December 2009
- ^ a b c d e «A very special tree house». Bio-pro.de. 4 February 2010. Archived from the original on 7 April 2014. Retrieved 14 April 2010.
- ^ a b US «A method of shaping a portion of a woody plant into a desired form is provided. The method is effected by providing a root of a woody plant, shaping the root into the desired form and culturing the root under conditions suitable for secondary thickening of the root.» 7328532, Golan, Ezekiel, «Method and a kit for shaping a portion of a woody plant into a desired form», issued 2008-02-12
- ^ a b c d e f Swati Balgi (September 2009), «Live Art» (PDF), Society Interiors Magazine, Prabhadevi, Mumbai: Magna Publishing, archived (PDF) from the original on 25 April 2011, retrieved 17 February 2011
- ^ a b c McKee, Kate (2012), «Living sculpture», Sustainable and water wise gardens, Westview: Universal Wellbeing PTY Limited, pp. 70–73
- ^ a b «Eco-Architecture Could Produce «Grow Your Own» Homes«. American Friends of Tel Aviv University. Archived from the original on 11 December 2009. Retrieved 6 May 2010.
- ^ a b c d e f g h i j k l Richard Reames (2005), Arborsculpture: Solutions for a Small Planet, Oregon: Arborsmith Studios, ISBN 0-9647280-8-7
- ^ Rodkin, Dennis (25 February 1996), The Gardener, Chicago Tribune Sunday
- ^ Oommen, Ansel (15 September 2013), The Artful Science of Tree Shaping, www.permaculture.co.uk, archived from the original on 12 November 2013, retrieved 6 November 2013
- ^ a b c d e f g h Richard Reames; Delbol, Barbara (1995), How to Grow a Chair: The Art of Tree Trunk Topiary, ISBN 0-9647280-0-1
- ^ a b c Roger, Fox (December 2012), «Artist tree», Better Homes and Gardens Last, p. 140
- ^ a b «Living Trees, Living Art». Archived from the original on 28 April 2009. Retrieved 8 May 2009.
- ^ a b Erlandson, Wilma (2001), My father «talked to trees», Westview: Boulder, p. 22, ISBN 0-9708932-0-5
- ^ a b Cattle, Christopher. «How to grow your stool». Archived from the original on 25 February 2009. Retrieved 14 June 2010.
- ^ a b Volz, Martin (October–November 2008), «A Tree shaper’s life.» (PDF), Queensland Smart Farmer, archived from the original (PDF) on 23 July 2011
- ^ a b c d Peter Cook; Becky Northey (2012). Knowledge to Grow Shaped Trees. Australia: SharBrin Publishing Ptd Ltd. ISBN 978-1-921571-54-1.
- ^ a b «Artists Shape Trees into Furniture and Art» (PDF), Farm Show Magazine, vol. 32, no. 4, p. 9, June–August 2008, archived from the original (PDF) on 8 March 2012, retrieved 8 May 2010
- ^ Weston, Sarah (3 October 2006), Axel Erlandson’s Tree Circus, Mid-County Post
- ^ a b c d e Hicks, Ivan; Rosenfeld, Richard; Whitworth, Jo (2007), Tricks with Trees, Pavilion Books, p. 160, ISBN 978-1-86205-734-0, archived from the original on 23 November 2020, retrieved 8 October 2020
- ^ «home & your garden», Going on a ‘bender’, Queensland Times, May 2012, p. 18
- ^ Zoë Hendon & Anne Massey (2019). Design, History and Time: New Temporalities in a Digital Age (first ed.). Great Britain: Bloomsbury Publishing Plc. ISBN 978-1-350-06066-1. Archived from the original on 23 November 2020. Retrieved 8 October 2020.
- ^ Jim Worrall (27 May 2007), Forest and Shade Tree Pathology: Wood Decay, archived from the original on 18 May 2011, retrieved 10 June 2011
- ^ a b c d e f «BOTANY BUILDINGS Grow Buildings From Trees!». 27 July 2009. Archived from the original on 6 March 2013. Retrieved 12 April 2013.
- ^ a b c d e Menges, Achim (2 March 2012). Material Computation ‘Higher Integration in Morphogenetic Design Architectural Design (Architectural Design)’. United Kingdom: John Wiley & Sons Ltd. p. 144. ISBN 978-0-470-97330-1. Archived from the original on 23 November 2020. Retrieved 8 October 2020.
- ^ a b Walpole, Lois (2004), grown home, archived from the original on 5 July 2010, retrieved 14 June 2010
- ^ a b c University of California, Cooperative Extension (November 2003), «Arborsculpture: Horticultural Art» (PDF), Landscape & Turf News, p. 6, archived from the original (PDF) on 10 June 2010, retrieved 12 December 2015
- ^ a b Ken Mudge; Jules Janick; Steven Scofield; Eliezer E. Goldschmidt (2009), Jules Janick (ed.), A History of Grafting (PDF), Issues in New Crops and New Uses, Purdue University Center for New Crops and Plants Products, orig. pub. John Wiley & Sons, Inc., pp. 442–443, archived (PDF) from the original on 15 June 2010, retrieved 13 May 2010 Note large file: 8.04MB
- ^ a b c d e f Cassidy, Patti (August 2008), «A Truly Living Art», Rhode Island Home, Living and Design Magazine, Swansea, Massachusetts: Home, Living & Design, Inc., pp. 26–27
- ^ a b c d e f Stephen Lesiuk. «BIOTECTURE II: PLANTBUILDING INTERACTION». Dept of Architecture, Sydney University. Archived from the original on 27 September 2011. Retrieved 14 May 2017.
- ^ Gillespie, Alison (October 2008). «Taking treehouses to whole new level». The Ecological Society of America. Retrieved 5 August 2021.
- ^ Thomas Vallas (25 May 2017). peer reviewer Luc Courard. «Using nature in architecture Building a living house with mycelium and trees». Frontiers of Architectural Research.
- ^ a b c d e f g Mack, Daniel (31 December 1996), Making Rustic Furniture: The Tradition, Spirit, and Technique with Dozens of Project Ideas (illustrated ed.), Lark Books, p. 160, ISBN 1-887374-12-4, archived from the original on 23 November 2020, retrieved 8 October 2020
- ^ a b «Only Natural Grown Chair». Shawano Leader Newspaper. Wisconsin Historical Society. 19 October 1922. Archived from the original on 12 November 2009. Retrieved 15 May 2010.
- ^ «Obituary of Axel Erlandson», Turlock Journal, p. 15, 30 April 1964
- ^ a b c d Link, Tracey (13 June 2008), «Senior project for Bachelor of Science degree in Landscape Architecture» (PDF), Arborsculpture: An Emerging Art Form and Solutions to our Environment, p. 41, archived from the original (PDF) on 25 February 2012
- ^ Wiechula, Arthur (1926) [1926], Wachsende Häuser aus lebenden Bäumen entstehend (Developing Houses from Living Trees), Verl. Naturbau-Ges, p. 320
- ^ a b «designboom:history of arborsculpture». Archived from the original on 20 November 2010. Retrieved 16 May 2010.
- ^ a b c d e f Ladd, Dan (22 January 2009), Sculpturefest 2008: Daniel Ladd, archived from the original on 27 July 2011, retrieved 14 June 2010
- ^ Extreme Nature: The Sculptures of Dan Ladd at Putney Library Archived 24 November 2010 at the Wayback Machine 10 October 2006.
- ^ Shaw, Kurt (11 August 2002), «Persephone Project promotes gardening as contemporary art medium», TribLiveNews, archived from the original on 15 June 2015, retrieved 30 June 2010
- ^ a b c Steve, Rhodes (6 April 2003), «No need to pull up a stump: Short of garden furniture?», Sunday Mail
- ^ a b «The father of Living Furniture», Bangkok Post, 16 January 1996
- ^ a b «Pooktre», Bricks & Mortar Magazine, 2008
- ^ «Pooktre«. Northey, Becky. Retrieved 5 May 2010.[permanent dead link] (Archived by WebCite at «Archived copy». Retrieved 2010-05-05. CS1 maint: url-status (link))
- ^ a b c McKie, Fred (20 April 2005), «Warwick artist grows wooden ‘jewels’ for World Expo», The Southern Free Times
- ^ Company profile: Arborsmith Studios, archived from the original on 22 September 2010
- ^ S. Okenga (2001), Eden on Their Minds: American Gardeners with Bold Visions, Clarkson Potter, p. 110, ISBN 0-609-60587-9
- ^ a b c d Nestor, James (February 2007), «Branching Out», Dwell, Dwell, LLC, p. 96, archived from the original on 21 May 2010, retrieved 15 June 2010
- ^ a b c d e Cattle, Christopher. «grown furniture home page». Christopher Cattle. Archived from the original on 26 December 2008. Retrieved 14 June 2010.
- ^ a b «Grown Furniture at the Museum of English Rural Life» (Press release). University of Reading, UK. 26 March 2008. Archived from the original on 23 December 2012. Retrieved 14 June 2010.
- ^ Cattle, Christopher. «grown furniture examples». Christopher Cattle. Archived from the original on 10 June 2010. Retrieved 14 June 2010.
- ^ a b Smolina, O O (2019). «Variability of approaches to arborsculptures». IOP Conference Series: Materials Science and Engineering.
- ^ a b «Five year deliveries», China Morning Business View, Farmington, Michigan: AccessMyLibrary, via CMP Information Ltd., via The Gale Group, 2003, retrieved 15 June 2010
- ^ a b c Treet Them Well, Chaotic Web Development, via ananova.com), 2 February 2005, archived from the original on 21 May 2010, retrieved 15 June 2010
- ^ Astrid, Paul (2013). «Building botany — Arbosculpture». Klimafarming-Garten an der Uni Tübingen.
- ^ Hoffman, Bill; Wire Services (3 February 2005), «Weird But True», New York Post (news ed.), p. 23, archived from the original on 3 November 2012, retrieved 15 June 2010
- ^ Hickey, Shane (2015), «The Innovators: growing solid wooden furniture without the joins», The Guardian
- ^ Sera, Corriere della (2015), «Gavin Munro: the essence of biodesign», Connetions
- ^ Munro, Gavin. «Harvesting chairs: How an English craftsman shapes furniture from the ground up». CBS News. Retrieved 8 June 2021.
- ^ Chan, Peter (1987), Bonsai Masterclass, Sterling Publishing Co., Inc., ISBN 0-8069-6763-3
- ^ Koreshoff, Deborah R. (1984), Bonsai: Its Art, Science, History and Philosophy, Timber Press, Inc., p. 1, ISBN 0-88192-389-3
- ^ a b Evans, Erv, Espalier, North Carolina State University Horticultural Science Department Cooperative Extension Service, archived from the original on 8 July 2010, retrieved 29 June 2010
- ^ The Complete Guide to Pruning and Training Plants, Joyce and Brickell, 1992, page 106, Simon and Schuster
- ^ John Seymour (1984). The Forgotten Arts A practical guide to traditional skills. Angus & Robertson Publishers. p. 54. ISBN 0-207-15007-9.
- ^ Coombs, Duncan; Blackburne-Maze, Peter; Cracknell, Martyn; Bentley, Roger (2001), «9», The Complete Book of Pruning (illustrated ed.), Sterling Publishing Company, p. 224, ISBN 978-1-84188-143-0
- ^ «A LIVING HOUSE – Terreform’s Fab Tree Hab». 18 September 2005. Archived from the original on 2 March 2013. Retrieved 14 April 2013.
- ^ a b «Grow your own home: ‘Fab tree hab’«. Archived from the original on 15 January 2016. Retrieved 14 April 2013.
- ^ a b c Karen Cilento (28 October 2011), «The Patient Gardener / Visiondivision», Arch Daily, Plataforma Networks, archived from the original on 31 December 2011, retrieved 8 March 2012
- ^ a b c d Foer, Joshua; Reames, Richard (Winter 2005–2006), «How to Grow a Chair: An Interview with Richard Reames», Cabinet Magazine, archived from the original on 7 January 2009, retrieved 15 May 2010.
- ^ Jules Janick (2009), Horticultural Reviews, vol. 35, John Wiley and Sons, p. 443, ISBN 978-0-470-38642-2, archived from the original on 23 November 2020, retrieved 8 October 2020
- ^ Jaya Jiwatram (25 August 2008), «We’re going to Live in the Trees», Popular Science Magazine, archived from the original on 5 August 2011, retrieved 10 June 2011
- ^ Hao Jinyao (11 May 2009), «The art of Tree shaping», Culture
- ^ Marras, Amerigo (1 February 1999), ECO-TEC: Architecture of the In-Between, Princeton Architectural Press, ISBN 978-1-56898-159-8, archived from the original on 23 November 2020, retrieved 8 October 2020
- ^ «Living Art». Discoveries. 6 September 2011. Archived from the original on 5 December 2010.
- ^ Varkulevicius, Jane (2010), Pruning for Flowers and Fruit, CSIRO Publishing, p. 96
- ^ Bunny Guinness (18 September 2011), «Train your trees into extraordinary shapes», Sunday Telegraph, UK/
- ^ Oommen, Ansel. «Baubotanik: The Botanically Inspired Design System That Creates Living Buildings.» ArchDaily, ArchDaily, 23 Oct. 2015, www.archdaily.com/775884/baubotanik-the-botanically-inspired-design-system-that-creates-living-buildings
- ^ «designboom: the alchemic force of the imagination transmutes nature». Archived from the original on 10 June 2011. Retrieved 10 June 2011.
- ^ Perréal, Jean (1516). «l’Alchimie». Musée Marmottan Monet. Archived from the original on 19 March 2009. Retrieved 8 May 2010.
- ^ Kamil, Neil (2005), Fortress of the Soul: Violence, Metaphysics, and Material Life in the Huguenots’ New World 1517–1751, JHU Press, pp. 384–385, ISBN 0-8018-7390-8, archived from the original on 23 November 2020, retrieved 22 February 2010
- ^ Swedenborg, Emanuel (2008) [1758], Earths in the Universe, BiblioBazaar, LLC, p. 104, ISBN 978-1-4375-3106-0, archived from the original on 23 November 2020, retrieved 8 October 2020
- ^ Lorber, Jakob (1995), Die Haushaltung Gottes (The Household of God) (Translation by Violet Ozols ed.), Lorber Verlag, p. 564, ISBN 978-3-87495-314-6
- ^ Tolkien, J. R. R. (October 2004), The Lord of the Rings, p. 1157, ISBN 9780618517657
Admin
Printable Plant Shape Matching Activity for Preschool Students
These printables are a clever and fun way for preschool students to learn about shapes and match them to the words for that shape.
There are six sheets total, with four having a different shape named upon each sheet (Square, Triangle, Circle, and Star) and space for three plants that have those shapes as the flower at the top to be placed upon the printable. The other two sheets feature 12 different plants that have different shapes atop the plant as a flower—three each of the four different shapes.
Preschool students can take each individual plant and match it with its flower to the correct sheet (e.g. putting the plant with a circle flower on the printable that says, «Circle,» and so forth). So that this activity can be done multiple times by many students everything can be laminated as well for easy cleaning and repeating.
Printable Plant Shape Pages
Below are the six total sheets, four with the individual shapes named and two with all of the plants that feature the plants with shape-based flowers.
How to Download the Files
This file package can be downloaded absolutely for free! Click here for details
Click Here for More Worksheets
- Home
- NZ Herald
- June 17, 2017
Thank you for visiting our website! Below you will be able to find the answer to Shaping (plant) crossword clue which was last seen on NZ Herald Crossword, June 17 2017. Our site contains over 2.8 million crossword clues in which you can find whatever clue you are looking for. Since you landed on this page then you would like to know the answer to Shaping (plant). Without losing anymore time here is the answer for the above mentioned crossword clue:
We found 7 possible solutions in our database matching the query Shaping (plant)
Possible Solution
P
R
U
N
I
N
G
«Shaping (plant)» in other crosswords:
| Puzzle | Solution |
|---|---|
| NZ Herald February 26 2021 | PRUNING |
| Metro September 6 2020 | PRUNING |
| NZ Herald March 15 2020 | PRUNING |
| Metro August 25 2019 | PRUNING |
| Metro May 14 2018 | PRUNING |
| NZ Herald June 17 2017 | PRUNING |
Want to know where PRUNING has appeared as a solution before? Click here for more information on that word.
Comments
Oh! It appears there are no comments on this clue yet. Would you like to be the first one?
Search Clues
Type in your clue and hit Search!
Crossword Solver
Fill the crossword solver with the word your are looking for. Use ‘?’ for unknown letters.
Words that start with
If you only have the first letter(s) of a word, type the letter(s) below.
or visit our complete Words that start with list.
Words that end with
If you only have the last letter(s) of a word, type the letter(s) below.
or visit our complete Words that end with list.
- Latest Clues
- Daily
- Random
- Comments
- Forum Discussions
- Ayuda con Crucigramas
Get the NZ Herald Crossword Answers delivered to your inbox every day!
Introduction
As sessile organisms, plants cannot escape from their habitat. They have to cope with the prevailing conditions, including abiotic factors like nutrient supply and biotic influences such as herbivory. Part of the adaptation strategy toward those challenges is an enormous degree of flexibility in plant architecture which is facilitated by the open, indeterminate development of plants. During plant embryogenesis, the apical-basal axis is established. At the poles of this axis, shoot and root apical meristems (SAM and RAM), respectively, develop as primary meristems. With the onset of post-embryonic development, the SAM extends the primary growth axis of the above-ground part of the plant. So-called phytomers are formed as repetitive basal modules of the plant shoot which consist of an internode and a node with one or more attached axillary leaves (Figure 1). In the leaf axils, secondary, lateral meristems are established and allow the formation of higher order morphological structures. The axillary meristems may develop a bud that can extend to form a branch, which constitutes a secondary growth axis. Branches are built up in the same way as the primary growth axis, and higher order branching can occur, leading to a complex structure. The architecture of a mature plant is therefore determined by the number and activity of axillary meristems and the growth characteristics of the branches that develop from axillary buds (Kerstetter and Hake, 1997; Sussex and Kerk, 2001; McSteen and Leyser, 2005; Schmitz and Theres, 2005; Bennett and Leyser, 2006; De Smet and Juergens, 2007; Janssen et al., 2014).
FIGURE 1. Illustration of plant architecture. Typical architecture of a dicot plant (A) and a monocot plant (B). The shoot apical meristem (SAM) establishes the shoot as the primary growth axis of the plant by continuously initiating phytomers, the basic modules of the plant shoot. A phytomer consists of an internode and a node with its attached leaf. In the leaf axils, axillary (secondary) meristems are formed in dicot and some monocot plants, which develop into an axillary bud and have the potential to continue growth to form an axillary branch. This branch can be regarded as a secondary growth axis and is built in the same way as the primary shoot. It can branch further to form higher-order branches (not shown). The primary root is established by its own meristem [root apical meristem (RAM)] and can also branch to form secondary or higher-order lateral roots. In addition to axillary branches, monocot plants can produce tillers which emanate from the base of the plant, which has extremely condensed internodes. The tillers form adventitious roots, called tiller nodal roots.
Axillary Meristem Initiation
Axillary meristems are the origin of lateral branches. They are formed in the center of the boundary zone at the adaxial side of the leaf base. The boundary zone separates the shoot apical meristem (SAM) from the developing leaf primordium. This zone is not just a border but fulfills an important function in meristem maintenance and organ development (Zadnikova and Simon, 2014). It is characterized by small cells, stiff cell walls and a low cell division rate. A key factor during establishment of the boundary zone is the transcription factor LATERAL ORGAN BOUNDARIES1 (LOB1) that induces the expression of BAS1, encoding a protein that has brassinosteroid inactivating activity (Bell et al., 2012). Brassinosteroids are plant steroid hormones that influence cell expansion and cell division (reviewed in Hardtke, 2007; Fridman and Savaldi-Goldstein, 2013). The LOB1-mediated decrease of brassinosteroid activity causes a reduction of cell size and cell division rate in the boundary zone compared to neighboring zones (Bell et al., 2012; Gendron et al., 2012). This effect is enhanced by the outward orientation of the auxin efflux carrier PIN-FORMED1 (PIN1) causing depletion of the plant growth hormone auxin in the boundary zone. During initial outgrowth of the leaf primordium, PIN1 is oriented toward the primordium. However, as the boundary zone develops, PIN1 is reoriented toward the SAM (Wang et al., 2014a,b). This reorientation depends on the kinase PINOID (PID) that controls basal-apical localization of PIN1 (Furutani et al., 2004). The importance of PIN reorientation and the role of PID in development of a functional boundary zone can be seen in pin1 and pid mutants that exhibit defects in axillary meristem formation (Wang et al., 2014a,b). Artificial increase of auxin in the developing boundary zone by localized expression of the auxin biosynthesis gene iaaM in transgenic Arabidopsis resulted in the lack of axillary meristems in a portion of the leaf axils (Wang et al., 2014a,b). On the contrary, boundary zone specific expression of a stabilized version of the AUX/IAA protein BODENLOS to reduce auxin signaling in this area resulted in the formation of axillary buds in the axils of cotyledons which was never observed in wild type plants (Wang et al., 2014a). Therefore, a local auxin minimum in the boundary zone appears to be important for axillary meristem formation.
Another gene having an effect on shoot lateral organ development is RPS10B, which was found in a suppressor screen of the more axillary branching2-1 (max2-1) mutant. It encodes the ribosomal protein S10e. Stirnberg et al. (2012a) discuss that in the mutant, levels of proteins, which are important for the regulation of auxin distribution and therefore auxin-mediated organ boundary patterning, may be imbalanced. Especially proteins with a high turnover rate, such as the Aux/IAA repressors involved in auxin signaling, may be affected by the ribosomal rps10b-1 mutation (Stirnberg et al., 2012a). In the same suppressor screen, FAR-RED ELONGATED HYPOCOTYL3 (FHY3) was found. The authors discuss this gene to be potentially involved in the regulation of auxin homeostasis, too (Stirnberg et al., 2012b).
Therefore, there appear to be many factors controlling the precise spatiotemporal auxin distribution during meristem development. In addition to auxin, Wang et al. (2014b) also discuss a role of cytokinin during AM initiation. They report a cytokinin pulse following and being dependent on the establishment of an auxin minimum in the boundary zone of the leaf axil and provide hints for the importance of cytokinin signaling during the establishment of the axillary meristem.
Tissue markers of the boundary zone are the Arabidopsis NAM-ATAF1/2-CUC2 (NAC) transcription factors CUP SHAPED COTYLEDONS1, 2, and 3 (CUC1, 2, and 3; Spinelli et al., 2011) that have redundant functions in meristem formation. In tomato, GOBLET (GOB) was identified as an ortholog of the CUC genes (Busch et al., 2011). Expression of these genes is a prerequisite for development of the SAM and the consecutive formation of the boundary zone. CUC genes are down-regulated by brassinosteroids. Thus, low brassinosteroid activity in the boundary zone not only reduces cell expansion and division as described above, but also allows the induction of CUC genes (Bell et al., 2012; Gendron et al., 2012).
The most pronounced difference between the SAM, the neighboring boundary zone and the developing leaf primordium is that cells in the SAM are kept in an indeterminate, non-differentiated state while cells of the boundary zone and the primordium differentiate. Meristematic identity of the SAM cells is retained by activity of the homeobox class I KNOX gene SHOOT MERISTEMLESS (STM; Long et al., 1996; Long and Barton, 2000). As soon as cells start to differentiate, STM is down-regulated by the MYB transcription factor AS1 and the LATERAL ORGAN BOUNDARY DOMAIN (LBD) transcription factor AS2 (Ikezaki et al., 2010). Interestingly, during an early phase of boundary zone development, STM continues to be transcribed in all cells of the boundary zone, albeit at a low level (Long and Barton, 2000). This indicates that, for a restricted time period, cells of the boundary zone keep the capacity to return to a meristematic stage. During this developmental phase, the axillary meristem is initiated (Grbic and Bleecker, 2000). A molecular marker of de novo axillary meristem formation is the focused and strong expression of STM in the center of the boundary zone. In Arabidopsis, this focused STM expression depends on the presence of the GRAS transcription factor LATERAL SUPPRESSOR (LAS; Greb et al., 2003). Orthologs of LAS are LS in tomato (Schumacher et al., 1999) and MONOCULM1 (MOC1) in rice (Li et al., 2003). Knockout mutants of LAS fail to develop axillary meristems during the vegetative stage (Greb et al., 2003). Keller et al. (2006) suggested that “LAS is required for reacquisition of indeterminate cell fate in axillary cells in the course of AM organization.”
Axillary meristem initiation and development is modulated by several factors that have partially redundant functions. In addition to LAS, the MYB factors REGULATOR OF AXILLARY MERISTEMS1 (RAX1) in Arabidopsis (Keller et al., 2006), as well as BLIND (BL) and POTATO LEAF (C) in tomato (Schmitz et al., 2002; Busch et al., 2011), influence axillary meristem development. Another factor is a basic helix-loop-helix (bHLH) protein called REGULATOR OF AXILLARY MERISTEM FORMATION (ROX) in Arabidopsis (Yang et al., 2012), LAX PANICLE1 (LAX1) in rice (Komatsu et al., 2001, 2003) and BARREN STALK1 (BA1) in maize (Ritter et al., 2002; Gallavotti et al., 2004).
For the ontogenetic origin of axillary meristems, two theories have been discussed (Sussex and Kerk, 2001). The de novo meristem formation theory is based on the fact that in some plant species, e.g., Arabidopsis, axillary meristems cannot be detected after leaf initiation by anatomical studies. In contrast, the detached or reserve meristem theory describes the situation in plants like tomato where meristematic cells from the SAM persist in the axils of newly built leaves and then, later during development, form axillary meristems (reviewed in Bennett and Leyser, 2006). However, the studies on LAS, RAX1, and ROX1 show that similar key factors control meristem initiation in plant species that seem to have contrasting mechanisms of meristem development. This indicates that axillary meristems in plants are generally formed by the same process. The fact that the boundary zone that just separated from the SAM continues to show STM expression argues for the detached meristem hypothesis. Cells of the boundary zone seem to be kept in a stage that is not fully determinate and, as a consequence, the axillary meristem can be initiated from this pool of cells. In conclusion, these data provide evidence that also in plants like Arabidopsis, where the meristem appears at later stages of development, the meristem is not formed de novo but built as a detached meristem (Leyser, 2003; Bennett and Leyser, 2006).
Axillary meristems strictly form on the adaxial side of leaf bases. This may be the reason why the transcription factor REVOLUTA (REV), that determines adaxiality, has been described as a further axillary meristem initiation factor (Otsuga et al., 2001). However, its effect on axillary meristem formation may be secondary and the primary function of REV is the control of radial patterning (Emery et al., 2003; Bennett and Leyser, 2006).
Activity of Apical Meristems and Control of Bud Outgrowth
The architecture of mature plants is determined by the frequency of axillary meristem initiation, the control of bud outgrowth, as well as subsequent dynamics in branch growth. Variation of these parameters generates the high morphological diversity observed in different plant species and even between individuals within a given species. This variation is largely based on genetic predisposition. However, the architecture that is characteristic of a plant species may be modified in response to environmental conditions. An important parameter of modification is the activity of axillary buds. Axillary branching is normally suppressed or at least reduced by the shoot apex through a regulatory system that has been termed apical dominance (reviewed in Cline, 1997; Leyser, 2005). The basic principles that govern bud outgrowth control have been described several decades ago. Snow (1925) could show that maintenance of apical dominance needs a signal that moves downward from a dominant shoot apex and, in addition, another signal may be transported upward into the dormant bud to suppress outgrowth. Thimann and Skoog (1933) identified the plant hormone auxin as the downward signal. Auxin, mainly synthesized in expanding young leaves of the plant apex (Ljung et al., 2001), is transported basipetally in the stem. Removal of the apical auxin source by decapitation abolishes apical dominance, while application of auxin to the apex of these decapitated plants can restore apical dominance (Thimann and Skoog, 1933). However, the inhibitory effect of auxin is not direct. It was shown that external auxin application to axillary buds does not prevent their outgrowth and experiments with radiolabeled auxin revealed that apex-derived auxin does not enter the dormant bud. Additionally, auxin transport appears to be too slow to mediate a direct effect (Hall and Hillman, 1975; Morris, 1977; Everat-Bourbouloux and Bonnemain, 1980; Booker et al., 2003). As a consequence of these studies, a long distance second messenger was postulated. According to this model, such a second messenger relays the downward auxin signal upward into the dormant bud. There are two good candidates for this messenger: cytokinins and strigolactones. Cytokinin is produced in roots and the stem and transported acropetally in the xylem (Nordstrom et al., 2004). Manipulations of plant cytokinin content show clear effects on bud outgrowth control, e.g., application of cytokinin to axillary buds releases dormancy even in plants that have an intact apex (Sachs and Thimann, 1964). Thus, with respect to bud outgrowth control, cytokinins act antagonistically to auxin. Most likely, the readout of auxin-cytokinin crosstalk generates part of the signaling chain that controls dormancy. The question of how auxin influences cytokinin as a second messenger was addressed by Nordstrom et al. (2004), who found that auxin can dampen cytokinin biosynthesis (Figure 2A). Basipetally transported auxin from the plant apex decreases expression of the cytokinin biosynthesis gene ISOPENTENYLTRANSFERASE (IPT) in the stem (Tanaka et al., 2006). In addition, it was shown for pea stems that auxin induces the cytokinin oxidase gene PsCKX2 (Shimizu-Sato et al., 2009). Cytokinin oxidases inactivate cytokinin and, thus, lower the pool of active cytokinin (Werner et al., 2001). As a consequence of decreased biosynthesis and increased degradation, the cytokinin content is lowered in the stem and bud dormancy is maintained. In contrast, the decrease of auxin in the stem after removal of the main auxin biosynthesis site by decapitation will lead to increased cytokinin biosynthesis (Bangerth, 1994; Figure 2B). In pea, the PsIPT1 and PsIPT2 genes are induced in the nodal stem near the axillary buds after decapitation. Consistently, increased cytokinin levels could be detected in excised nodal stems (Tanaka et al., 2006). Cytokinin may then be transported into the adjacent buds. Indeed, it was shown in pea that the zeatin riboside content increased in axillary buds after decapitation (Turnbull et al., 1997).
FIGURE 2. Schematic illustration of different pathways and models in the control of bud outgrowth. In an intact plant (A), the apex is a strong auxin source. Auxin is transported basipetally in the polar auxin transport stream (PATS). According to the second messenger model, auxin promotes strigolactone (SL) and represses cytokinin (CK) biosynthesis, respectively. Both hormones have adverse effects on bud outgrowth, most likely acting via the transcription factor BRANCHED1/TEOSINTE BRANCHED1 (BRC1/TB1). Auxin indirectly promotes BRC1/TB1 expression, which suppresses bud outgrowth. GRASSY TILLERS1 (GT1) is a putative downstream target for TB1 in monocots. According to the auxin transport canalization model, the axillary bud is also an auxin source and as a prerequisite for vascular tissue formation and bud outgrowth, it has to establish its own auxin export. However, it competes with the shoot apex for the stem as a shared auxin sink. This competition is enhanced by SL, which reduces plasma membrane accumulation of the PIN1 auxin efflux carrier and therefore inhibits the PATS in the main stem. High auxin levels in the stem prevent the formation of an initial auxin export flux from the bud, and therefore suppress bud outgrowth. After decapitation (B), the apex as the primary auxin source is removed. Biosynthesis of SL is not promoted anymore, while repression of CK biosynthesis is released. Furthermore, the auxin level in the main stem is reduced and thus the sink capacity is increased, facilitating the establishment of an initial auxin export from the bud. After bud outgrowth, the emanating branch takes over the function of the lost apex as the primary auxin source and re-establishes apical dominance. Both described models, the second messenger model and the auxin transport canalization model, are not mutually exclusive, and the described pathways could contribute to bud outgrowth control simultaneously. After bud outgrowth, the angle of the branch is also under control. TILLER ANGLE CONTROL1 (TAC1) increases the tiller angle in monocots, while LAZY1 has the opposite function and reduces the tiller angle. Black lines and letters designate active pathways; light gray lines and letters indicate suppression or down-regulation of the respective pathway.
In addition to damages to the apex, other environmental impacts such as nutrient availability (e.g., nitrogen or phosphorus concentrations in the soil) or planting density-related shading, also profoundly change plant architecture (Casal et al., 1986; Lopez-Bucio et al., 2002; Yoneyama et al., 2013; de Jong et al., 2014). The developmental response of the plant shoot to nutrient supply most likely involves a long distance, graft-transmittable signal from the root. Root tips are a main biosynthesis site of cytokinin (Miyawaki et al., 2004; Nordstrom et al., 2004) and it is tempting to speculate that changes in the cytokinin export from the root to the shoot via the xylem stream provide the postulated long distance signal for root–shoot communication. However, Faiss et al. (1997) showed in grafting experiments that transgenic roots overproducing cytokinin could not induce bud outgrowth in wild type scions, which made cytokinin unlikely to be the elusive signal. Analyses of branching mutants in Arabidopsis (more axillary branching -max), pea (ramosus —rms), petunia (decreased apical dominance—dad), and rice (dwarf –d) finally led to the discovery of the shoot branching hormone strigolactone (SL; Gomez-Roldan et al., 2008; Umehara et al., 2008) which features the required characteristics of the sought-after long distance signal in branching control: it inhibits shoot branching (Gomez-Roldan et al., 2008; Umehara et al., 2008), it can be transmitted from wild type roots to mutant shoots via grafting and complements the branching phenotype (Beveridge et al., 1997; Sorefan et al., 2003; Booker et al., 2005; Beveridge, 2006). Acropetal SL transport was shown to occur in the xylem (Kohlen et al., 2011) and the biosynthesis is increased by auxin (Sorefan et al., 2003; Figure 2A).
After the discovery of SLs as branching hormones, much effort was put in unraveling their biosynthesis and signaling pathways. SL biosynthesis starts from carotenoid precursors via the action of the all-trans/9-cis-β-carotene isomerase D27 in rice and AtD27 in Arabidopsis (Lin et al., 2009; Waters et al., 2012). Subsequent processing is carried out by the carotenoid cleavage monooxygenases CCD7 and CCD8. These enzymes are known in several species and named MAX3 and MAX4 in Arabidopsis (Sorefan et al., 2003; Booker et al., 2004), RMS5 and RMS1 in pea (Sorefan et al., 2003; Johnson et al., 2006), D17 and D10 in rice (Ishikawa et al., 2005; Arite et al., 2007; Alder et al., 2012), and DAD1 in petunia (Snowden et al., 2005).
Successful complementation of Arabidopsis max mutants with putative MAX orthologs from willow and poplar and response of willow buds to the SL analog GR24 indicate that SLs are also synthesized and perceived in woody plants such as trees (Ward et al., 2013; Czarnecki et al., 2014).
In addition to CCD7 and CCD8, the cytochrome P450 monooxygenase MAX1 is involved in downstream SL biosynthesis in Arabidopsis (Stirnberg et al., 2002; Booker et al., 2005; Table 1). An overview about all aforementioned SL biosynthesis genes can be found in Table 1.
TABLE 1. Genes involved in strigolactone biosynthesis and signaling.
Strigolactone biosynthesis generally occurs in roots and in the shoot (Auldridge et al., 2006; Umehara et al., 2008; Mashiguchi et al., 2009). Grafting studies revealed that wild type rootstocks can suppress the phenotypes of max1, max31, and max4 biosynthesis mutant scions (Sorefan et al., 2003), indicating that SL or a SL precursor can travel from root to shoot (Turnbull et al., 2002; Beveridge, 2006). The product of the CCD8 reaction, carlactone, has been discussed as a possible mobile SL precursor (Seto and Yamaguchi, 2014). This hypothesis is based on the observation that a max1 rootstock can complement the branching phenotype of a max4 scion (Booker et al., 2005). Recently, Abe et al. (2014) analyzed the MAX1 reaction in SL biosynthesis in detail. They demonstrated that carlactone is converted to carlactonic acid by the action of MAX1 in Arabidopsis. However, subsequent reactions to generate bioactive SLs remain to be elucidated. The same authors reported hints for the interaction of a carlactonic acid methyl ester with the putative SL receptor AtD14 (see SL signaling discussion below; Abe et al., 2014). Therefore, we are close to fully understanding the biosynthesis pathway of at least one bioactive SL. However, there are multiple other bioactive variants of SLs. The detailed reactions leading to this diversity as well as possible alternative biosynthesis pathways remain to be discovered.
Grafting experiments also revealed a class of SL response mutants that could not be complemented by wild type rootstocks (Beveridge et al., 1996; Stirnberg et al., 2007), indicating a role in SL perception and signaling rather than biosynthesis. An example is the Arabidopsis max2 mutant (Stirnberg et al., 2007) that encodes an F-box protein involved in SL signaling. Its counterparts in rice and pea were described previously (Ishikawa et al., 2005; Johnson et al., 2006; Table 1). Within 6 years after the first description of SLs as branching hormones, further key components of SL signaling have been identified and a tentative scaffold of the signal transduction pathway has been assembled (Bennett and Leyser, 2014; Waldie et al., 2014). The α/β hydrolase D14 is most likely a receptor for SL. d14 mutants in Arabidopsis, petunia and rice are insensitive to treatment with the SL analog GR24 and show an increased branching phenotype. Also, D14 exhibits high and specific affinity to GR24 (Kagiyama et al., 2013). In the presence of GR24, the petunia D14 ortholog DAD2 interacts with PhMAX2 (Hamiaux et al., 2012). This indicates that in analogy to other plant hormone signaling pathways, D14 may interact with the F-box protein MAX2 upon SL binding, leading to ubiquitination-mediated degradation of a SL signaling repressor (Bennett and Leyser, 2014). Since max2 mutants show pleiotropic effects, it is likely that MAX2 interacts with several pathways and may mediate degradation of different target proteins. Indeed, three different candidate repressors for the strigoalactone signaling pathway have been identified: DELLA proteins (Nakamura et al., 2013), BES1 (Wang et al., 2013), and D53 in rice (Jiang et al., 2013; Zhou et al., 2013). While interaction of MAX2 with DELLA proteins and BES1 may point to cross talk with the gibberellic acid and brassinosteroid pathway, respectively, D53 emerges as the genuine SL pathway repressor (reviewed in Bennett and Leyser, 2014). Dominant gain of function mutations in D53 prevent SL-mediated degradation of the protein and shut off SL signaling. Moreover, rice D53 interacts with D3, which is the rice ortholog of MAX2, and d3 mutants are suppressed by knockdowns of D53 (Jiang et al., 2013; Zhou et al., 2013). A possible Arabidopsis ortholog of D53 is SUPPRESSOR OF MORE AXILLARY GROWTH2 LIKE 7 (SMXL7; Stanga et al., 2013; Bennett and Leyser, 2014; Table 1). Interestingly, the basic principle of SL signaling is similar to auxin, jasmonic acid, and gibberellic acid signaling (reviewed in McSteen and Zhao, 2008). Briefly, binding of the hormone to a receptor activates an F-box protein-containing SCF E3 ligase complex, which mediates ubiquitination and subsequent degradation of a transcriptional repressor. Ultimately, this leads to changes in transcription of a specific set of genes (Hagen and Guilfoyle, 2002; Hartweck, 2008; Memelink, 2009).
Summarized, cytokinin and SL were shown to regulate bud outgrowth, but the mechanism of bud dormancy control and the reciprocal effect of these plant hormones had to be integrated into a model. Prusinkiewicz et al. (2009) and Balla et al. (2011) suggested combining the second messenger model with a model introduced by Li and Bangerth (1999). Their model of “autocorrelative inhibition” is based on the auxin canalization hypothesis by Sachs (1981) and discusses a competition of buds for establishment of a polar auxin transport stream (PATS). The auxin canalization hypothesis (reviewed in Domagalska and Leyser, 2011) suggests a feed forward mechanism to explain the establishment of polar auxin transport routes that induce the development of vascular tissues. Starting from an auxin source that provides a high auxin concentration, competent cells will transport auxin away from the source and establish an auxin gradient across the tissue. From this initial auxin flow, continuous transport will build up, keeping a high auxin concentration in the transport competent cells and subsequently increasing the expression and polarization of auxin carriers in these cells. As a consequence, auxin transport will further strengthen in a feed forward loop, which sustains and enhances transport competence in files of specific cells. Along these transport routes, vascular tissue will differentiate.
Research on the PIN auxin efflux carrier proteins provided experimental support for the canalization model. Biosynthesis and plasma membrane localization of PIN proteins are elevated by auxin (Paciorek et al., 2005) and the expression of PIN proteins precedes vascular development (Sauer et al., 2006; Scarpella et al., 2006; Wenzel et al., 2007). This model can be adapted for a hypothesis on the mechanisms that control apical dominance. As an initial auxin gradient is a prerequisite for the development of a PATS, only buds that achieve to build up an auxin gradient between the bud as an auxin source and the stem as a common auxin sink have the ability to establish a PATS and grow out. Usually, the actively growing apex is the main auxin source (Figure 2A). According to the auxin canalization model, apical dominance is therefore exerted by the apex through saturation of the auxin transport capacity of the stem, acting as an auxin sink. As a consequence, axillary buds are prevented from successfully establishing an initial auxin flux. Hence, they remain dormant.
After removal of the dominant apex, e.g., by decapitation, the auxin level in the stem decreases. The resulting increase in the sink capacity of the stem facilitates an initial auxin flux from dormant buds into the stem, finally releasing the dormancy of buds in the neighborhood of the formerly dominant shoot tip (Figure 2B). As soon as one or few buds grow out, the growing branches re-establish apical dominance by exporting auxin to the main stem. The sink capacity of the stem is consequently reduced back to normal levels, preventing further dormant buds from growing out.
Both models, the second messenger model and the model of autocorrelative inhibition/auxin canalization, are complementary. Cytokinin and SL, respectively, influence sink strength of the stem through changes in auxin biosynthesis and modification of PATS. As a consequence of decapitation, the inhibitory effect of auxin on cytokinin biosynthesis is dampened and increased cytokinin levels might enhance local auxin biosynthesis in the bud, increasing its auxin source strength. At the same time, the sink capacity of the stem may be enhanced by a cytokinin-mediated induction of the PATS in the stem by increased synthesis and polarization of PIN auxin efflux carriers. Indeed, such increased expression and polarization was shown for PsPIN1 in axillary buds after external cytokinin application (Kalousek et al., 2010). Furthermore, Marhavy et al. (2014) postulated a role for cytokinin in modulating AtPIN1 abundance and polarization during lateral root organogenesis.
In contrast to cytokinin, SL appears to decrease the amount of the PIN auxin efflux carrier at the membrane and, thus, lower auxin transport capacity in the stem. This was observed in stems of Arabidopsis SL-pathway mutants, which showed increased AtPIN1 levels as well as an increased auxin transport (Bennett et al., 2006; Prusinkiewicz et al., 2009). According to the auxin transport canalization model, SLs will, therefore, aggravate the establishment of auxin export from axillary buds, leading to increased apical dominance (Prusinkiewicz et al., 2009). Decapitation triggers down-regulation of SL biosynthesis gene CCD8 transcript levels (Foo et al., 2005), most likely resulting in reduced SL biosynthesis. Such a reduction of SL levels would cause a release from their antagonistic effect on PIN polarization. As a result, an increased auxin flux to the root would occur and, thus, further increase the sink capacity of the stem. Summarized, high cytokinin and low SL levels may increase source strength of the bud and increase sink capacity of the stem, and, thus, facilitate the successful establishment of an auxin gradient. This gradient would allow an initial auxin flow from the bud to the stem and the establishment of vascular tissue as a prerequisite for bud outgrowth (Figures 2A,B). Already Sorokin and Thimann (1964) observed that a vascular connection between axillary buds and the main stem coincides or precedes bud outgrowth.
A drawback of the hypotheses on apical dominance control by auxin is the discrepancy between auxin transport velocity and bud outgrowth kinetics after decapitation. In decapitated pea plants, buds start to grow out before auxin concentrations in the associated nodal stem are diminished due to removal of the apical auxin source (Morris et al., 2005). Thus, an alternative primary messenger is discussed. Mason et al. (2014) reported that after decapitation, sucrose concentrations in axillary buds increased. Moreover, buds could be released from dormancy by sucrose treatment and inhibition of sucrose transport by girdling prevented outgrowth of buds. Importantly, the measured speed of sucrose transport is sufficient to relay the signal from the shoot apex to a dormant axillary bud in time before first signs of bud outgrowth occur. Mason et al. (2014) therefore suggest that the primary signal after decapitation is sucrose and that auxin controls the number of buds that will grow out. The observation that the branching suppressor BRANCHED1 (BRC1) is down-regulated after sucrose treatment provides further arguments for this “nutritive hypothesis,” whose general concept was postulated earlier (reviewed in Phillips, 1975).
BRANCHED1 is a Key Factor in Bud Outgrowth Control
BRANCHED1 (BRC1) is a TB1 CYCLOIDEA PCF (TCP) type transcription factor (Aguilar-Martinez et al., 2007; Finlayson, 2007). Proteins of this group are either assigned to class I which contains PCF-like proteins or class II which consists of CYCLOIDEA/TB1-like proteins. It has been suggested that class I TCP factors increase cell division rates, while class II TCP factors inhibit cell cycle progression (Martin-Trillo and Cubas, 2010). The protein group takes its name from the TCP domain which is a highly conserved 59 amino acid basic helix-loop-helix structure that mediates DNA binding, protein–protein interaction, and nuclear targeting. Class II TCP transcription factors that regulate axillary meristem activity have been identified in several plant species (Doebley et al., 1997; Takeda et al., 2003; Kebrom et al., 2006, 2010; Aguilar-Martinez et al., 2007; Finlayson, 2007; Minakuchi et al., 2010; Martin-Trillo et al., 2011; Braun et al., 2012). Even slight expression changes of these factors profoundly modify plant architecture, as it was described for TB1 levels in maize compared to its anticipated ancestor teosinte (Doebley et al., 1997). Orthologs of maize TB1 were identified in other monocots like rice (FINE CULM1/OsTB1) and sorghum (SbTB1; Takeda et al., 2003; Kebrom et al., 2006). Aguilar-Martinez et al. (2007) and Finlayson (2007) described the TB1 orthologs BRANCHED1 (BRC1= TCP18) and BRANCHED2 (BRC2= TCP12) in the dicot species Arabidopsis. The fact that Arabidopsis contains two BRC paralogs is due to duplications of the Arabidopsis genome (Franzke et al., 2011; Vanneste et al., 2014). With respect to axillary branching, BRC1 seems to be the major regulator, while BRC2 shows a comparably low expression and brc2 knockout lines exhibit weaker phenotypes compared to brc1 plants (Aguilar-Martinez et al., 2007; Finlayson, 2007). BRANCHED1 genes were also identified in tomato (SLBRC1a and b; Martin-Trillo et al., 2011) and pea (PsBRC1; Braun et al., 2012). In accordance with BRC1 being a suppressor of branching, brc1 knockout mutants have more rosette branches. While in wild type Arabidopsis plants less than 40% of buds grow out, almost 100% of rosette buds elongate and form a branch in brc1 plants (Aguilar-Martinez et al., 2007). In addition, leaf axils of cotyledons in brc1 plants sometimes develop axillary meristems that form buds and grow out. In contrast, leaf axils of cotyledons never develop axillary buds in wild type plants. This indicates that BRC1 not only controls bud outgrowth, but also regulates axillary meristem initiation. Leaf axils of cauline branches (shoots of the inflorescence) are not affected in brc1 knockout lines. Thus, BRC1 specifically controls axillary meristem initiation and bud outgrowth in rosette leaf axils.
The BRC1 expression pattern correlates well with the anticipated role of BRC1 as a repressor of cell division and bud outgrowth. As revealed by Northern Blot, qPCR and in situ hybridization experiments, BRC1 expression is high in dormant rosette leaf buds and low in elongating, i.e., growing buds (Aguilar-Martinez et al., 2007; Finlayson, 2007). In addition, a gradient of BRC1 expression exists along the apical to basal axis in rosette leaf buds of Arabidopsis grown under long day conditions. Young buds near the shoot apex exhibit low BRC1 expression levels and older buds at the base of the rosette contain high amounts of BRC1 transcript (Finlayson, 2007). This coincides with the basipetal wave of axillary bud initiation and outgrowth in Arabidopsis after onset of flowering, i.e., buds with lower basal BRC1 levels grow out earlier (Hempel and Feldman, 1994). In other investigated tissues than buds, BRC1 transcript levels are very low or non-detectable (Aguilar-Martinez et al., 2007; Finlayson, 2007), emphasizing its specific role in the regulation of bud outgrowth.
In order to investigate its subcellular localization, Aguilar-Martinez et al. (2007) expressed BRC1 as GFP fusion under the control of the constitutively and ubiquitously active 35S promoter and showed that BRC1 is localized in the nucleus. With these p35S:GFP:BRC1 plants, they observed a severely stunted growth phenotype (Aguilar-Martinez et al., 2007), which is probably the result of misexpression of BRC1 at the shoot apex, further underlining its role as a growth repressor. Taken together, these observations indicate that in dicots, BRC1 acts as a transcriptional regulator that inhibits cell division in axillary buds. It was suggested that a final target of the signaling chain that involves TB1/BRC1 may be factors like PCNA that regulate the cell cycle (Müller and Leyser, 2011).
Expression of maize TB1 in wheat from its native maize promoter (Lewis et al., 2008) or OsTB1 in rice using the strong and constitutive rice actin promoter (Takeda et al., 2003) did not decrease plant growth but specifically affected outgrowth of axillary buds. Investigations by Guo et al. (2013) indicate that in monocots, TB1 may have a different mode of action than in dicots and may explain why rice OsTB1 overproducers do not show growth depression. Guo et al. (2013) identified the MADS box factor OsMADS57 that functions to increase tillering. Tillers are axillary branches that originate from the shoot base of monocots (Figure 1B). OsMADS57 is a transcriptional repressor that down-regulates expression of the SL receptor DWARF14. TB1/BRC1 in turn directly interacts with the OsMADS57 protein and, thereby, inactivates OsMADS57. As a consequence DWARF14 expression is de-repressed and SL perception is increased. Thus, in monocots TB1/BRC1 may not repress progression of the cell cycle, but control outgrowth of axillary buds by enhancement of SL signaling.
BRANCHED1 is a Central Integrator of Endogenous and Environmental Factors that Modulate Branching
Endogenous Factors/Hormonal Regulation
In order to investigate a possible influence of auxin on BRC1, Aguilar-Martinez et al. (2007) and Finlayson (2007) analyzed BRC1 expression in rosette buds of 35S:YUCCA plants that exhibit increased apical dominance due to auxin overproduction. Aguilar-Martinez et al. (2007) reported no effect of increased auxin levels on BRC1 expression in these plants. However, Finlayson (2007) determined BRC1 expression in upper and lower buds separately and found a significant increase in upper buds of 35S:YUCCA plants compared to wild type plants. Therefore, auxin seems at least partially to play a role in influencing BRC1 expression. Direct application of cytokinin on buds reduced BRC1 transcript levels in pea (Braun et al., 2012; Dun et al., 2012). Also in rice, cytokinin application decreased FINE CULM1 (FC1) expression (Minakuchi et al., 2010). In accordance with these observations Arabidopsis altered meristem program1 (amp1) mutants, which show increased cytokinin levels, exhibit slightly decreased BRC1 expression and more branches than wild type plants (Aguilar-Martinez et al., 2007).
The strongest effect on BRC1 transcript levels was observed in max1, max3, max4 SL biosynthesis mutants. The down-regulation of BRC1 expression in Arabidopsis max mutants indicates that SLs regulate BRC1 transcriptionally (Aguilar-Martinez et al., 2007). Data in favor of the hypothesis that BRC1 acts downstream of SLs has also been obtained from investigations in pea. Studies showed that PsBRC1 transcript levels are upregulated by SL application and down-regulated in SL synthesis and signaling mutants (Braun et al., 2012; Dun et al., 2013). In turn, rice fc1 knockout mutants did not respond to SL (Minakuchi et al., 2010) and also in Arabidopsis, GR24 treatment did not repress the increased branching phenotype of the Atbrc1 mutant (Brewer et al., 2009). In contrast, overexpression of FC1 could not suppress the branchiness of SL mutants (Minakuchi et al., 2010) and FC1 expression remains high in buds of SL mutants (Arite et al., 2007). These results appear to be contradicting and may be explained by other branching pathways in which SLs are involved (e.g., modulation of auxin transport, see auxin canalization model) as well as the fact that BRC1 is not solely regulated by SLs. BRC1 was proposed to be a central integrator of different branching pathways (Aguilar-Martinez et al., 2007).
Summarized, there appears to be an effect of the three main branching hormones auxin, cytokinin and SL on BRC1 (Figure 2A and Table 2), and further pathways seem to play a role (Rameau et al., 2015).
TABLE 2. Regulation of BRC1/TB1 expression.
Exogenous Factors/Shading
The signaling chains of auxin, cytokinin, and SL are modulated by environmental factors like shading or the plant nutritional status. The level of shading by neighboring plants is a measure for population density and, thus, an indicator of competition for light. Red light is absorbed by plants, while far red light is largely transmitted through the leaf canopy. As a consequence, shading by other plants reduces the ratio of red light to far red light (R/FR). Plants quantify this ratio through the phytochrome system and react with the shade avoidance syndrome that enables plants to outgrow the competitors for light (Casal, 2013; Pierik and de Wit, 2014). By suppression of branching, more resources are allocated to the main shoot and, consequently, the growth rate of the shoot increases and the plants grow taller in a shorter period of time.
The photoreceptor phytochrome can adopt two different conformations: Pr and Pfr. Upon absorption of red light, Pr (inactive) is converted to Pfr (active) which shuttles to the nucleus and controls gene expression through interaction with PHYTOCHROME INTERACTING FACTOR (PIF) or PIF3-like (PIL; Leivar and Monte, 2014) Within the family of five phytochromes in Arabidopsis, mainly phyB was shown to control red light responses of plant architecture (Finlayson et al., 2010; Reddy and Finlayson, 2014). In sorghum, low R/FR ratios or knockout of phyB prevented bud outgrowth, which was correlated with high TB1 transcript levels in axillary buds (Kebrom et al., 2006). It was hypothesized that phyB suppresses TB1/BRC1 and that the high FR proportion of light in a dense plantation will convert active phyB Pfr to inactive phyB Pr and thus, suppress bud outgrowth via increased TB1/BRC1 expression. Similarly, knockout of phyB increases TB1/BRC1 levels and therefore, reduces bud outgrowth. The observation that the Arabidopsis knockout mutant brc1-2 does not show branching suppression under low R/FR conditions supports the hypothesis that TB1/BRC1 plays a central role in branching suppression during shade avoidance (Gonzalez-Grandio et al., 2013). A putative downstream target of TB1 during the shade avoidance response in maize is the HOMEODOMAIN-LEUCINE ZIPPER (HD-ZIP) protein GRASSY TILLERS1 (GT1; Whipple et al., 2011). GT1 is expressed in leaf primordia of axillary buds and in provascular tissue below the axillary bud. Interestingly, signals of GFP-tagged GT1 were observed in cells of the axillary meristem, indicating non-cell-autonomous activity of GT1. Comparable to tb1 loss-of-function mutants, gt1-1 knockout mutants exhibit an increased branching phenotype. The significantly reduced GT1 expression in tb1 mutants indicates that TB1 and GT1 act in the same pathway. Since TB1 expression is not changed in gt1 mutants, it is likely that TB1 acts upstream of GT1 and regulates its expression (Figure 2A). Light conditions with a low R/FR ratio induce the expression of GT1, indicating that suppressed branching during the shade avoidance syndrome is due to TB1-mediated upregulation of GT1 expression.
Plants that suffer from suboptimal nutrient supply also exhibit decreased branching comparable to plants that compete for light. However, in contrast to the shade avoidance syndrome, during nutrient deprivation resources are not allocated to the shoot but instead to the root to facilitate enhanced nutrient uptake from the soil. Nutrient-induced changes in shoot/root ratio and root development are most obvious with plants grown under phosphate deficiency (Forde and Lorenzo, 2001; Lopez-Bucio et al., 2002). Branching in these plants is suppressed and many lateral roots develop near the soil surface, which was termed “topsoil foraging” (Peret et al., 2014). These changes in root morphology increase phosphate uptake from soil layers that are enriched in phosphate (Peret et al., 2011; Niu et al., 2013; Hunter et al., 2014).
Kohlen et al. (2011) quantified the number of shoot branches of Arabidopsis wild type and SL biosynthesis (max1, max4) and signaling (max2) mutants under phosphate sufficient and phosphate deficient conditions. Branching of wild type plants was significantly reduced under phosphate deficiency while none of the max mutants responded to low phosphate. The observed difference in branching suppression correlated with the SL content of the xylem sap. The strigolactone orobanchol could be detected in root exudates and xylem sap of wild type Arabidopsis and showed an increase in concentration when Arabidopsis was grown on phosphate deficient substrate. In contrast, the root exudate of the SL biosynthesis mutants max1 and max4 that were unresponsive to phosphate deficiency did not show an increase in SL content under phosphate-limiting conditions (Kohlen et al., 2011). Phosphate starvation increased SL synthesis also in tomato (Lopez-Raez et al., 2008), sorghum (Yoneyama et al., 2007), and rice (Umehara et al., 2010). Umehara et al. (2010) showed that the rice SL biosynthesis genes D17 (MAX3 in Arabidopsis) and D10 (MAX4 in Arabidopsis) are induced by low phosphate conditions.
Similarly to phosphate, also the nitrogen supply influences plant architecture. Low nitrogen suppresses branching and changes the root/shoot ratio toward higher root biomass proportions (Forde and Lorenzo, 2001; Euring et al., 2012). Quantification of SLs in roots and root exudates of sorghum and pea plants grown under low nitrogen conditions showed that nitrogen deficiency increased SL levels in these plants (Yoneyama et al., 2007; Foo et al., 2013), which points to SL-mediated suppression of bud outgrowth under nitrogen limitation. Vice versa, optimal nitrogen supply decreases SL production, which may lead to increased branching (Yoneyama et al., 2013).
A hallmark of SL activity is the decrease of auxin transport in the main stem via decrease of PIN1 levels at the plasma membrane, as mentioned earlier (Prusinkiewicz et al., 2009). However, auxin transport capacity in the main stem of Arabidopsis is not diminished by low nitrogen (de Jong et al., 2014), but, instead, auxin supply to the PATS from the main shoot apex is higher in nitrogen starved plants. The increased auxin biosynthesis at the apex makes it a stronger auxin source, reducing the sink strength of the PATS relative to the axillary buds. According to the canalization hypothesis (Sachs, 1981), this weak sink strength will prevent establishment of a PATS from axillary buds and, thus, consolidate bud dormancy. Analyses of Arabidopsis mutants showed that intact auxin signaling and SL biosynthesis are both required for increased supply of auxin from the shoot apex leading to suppression of branching under nitrogen starvation (de Jong et al., 2014).
In conclusion, phosphate and nitrogen supply of the plant clearly affect plant architecture and SLs are involved in the plant responses to nutrient supply. However, the mechanism leading to a change in branching may vary in different plant species.
The nutrient- or shading-induced changes in plant architecture exemplify that plants can adapt their branching patterns to the prevailing environmental conditions. This demonstrates that plant architecture closely correlates with plant growth and survival. Likewise, crop plant performance is determined by branching characteristics and it is not surprising that during domestication of crop plants, certain architectural traits were a major target for selection of improved cultivars. Especially monocot crops like rice, sorghum, maize, and wheat are of great importance for world nutrition. The architectural diversity of monocot plants allowed the selection of specific architectural traits from a broad natural gene pool during domestication.
Branching Relevant Genes Selected during Domestication and Plant Breeding
Monocot crop plants belong to the grasses which have been assigned to two major clades, consisting of subfamilies (Barker et al., 2001). Cereals of the first clade, which are important for world nutrition, belong to the subfamily Ehrhartoideae (including rice) and the Pooideae (including oat, wheat, barley, rye). Within the separate, second major clade is the subfamily of the Panicoideae with maize, sorghum, and millets. Grasses exhibit two types of vegetative branching patterns (Doust, 2007), depending on the position of branch development with respect to the plant main axis. Tillers are typical for many grasses and determine their characteristic growth habit (Figure 1B). Tillers are branches that originate from nodes near the plant basis. These branches reach a similar height like the main stem and have the capacity to form adventitious roots. Axillary branches that initiate at upper positions of the culm (the main stem of grasses) are similar to branches of dicot plants. Grasses of the two major phylogenetic clades can be classified according to these branching patterns. Plants of the Ehrhartoideae and the Pooideae develop many tillers and no axillary branches while members of the Panicoideae produce tillers and, in addition, initiate axillary meristems that can grow into axillary branches (Doust, 2007).
The architectural traits selected during the domestication of crop plants include the extent of vegetative shoot and inflorescence branching, branch angle, as well as internode elongation. Inflorescence branching and genes involved in stem elongation like the DELLA genes (Peng et al., 1999; Sasaki et al., 2002) have been covered in recent reviews (Fernandez et al., 2009; Teo et al., 2014; Zhang and Yuan, 2014). Here, we will therefore focus on vegetative branching and branch angle.
Changes in vegetative branching phenotypes during plant domestication are most evident in monocot crop plants and the molecular bases of these changes have been thoroughly studied. During domestication of panicoid grasses, plant lines have been selected that show a decrease in both tillering and axillary branching. Modern cultivars of domesticated maize plants develop ideally only one female inflorescence (ear) and a high proportion of fixed carbon is allocated to the developing ear. Only the main stem terminates in a single male inflorescence (tassel). In contrast, wild forms of Zea mays subsp. mays (Zea mays subsp. parviglumis and Zea mays subsp. mexicana, collectively named teosinte) develop many axillary branches at the main stem which produce female inflorescences from secondary axillary meristems. Each branch terminates in a male inflorescence. Doebley et al. (1997) discovered that one of the quantitative trait loci that determine maize architectural changes during domestication carries the TEOSINTE BRANCHED1 (TB1) gene. Small changes in expression strength of TB1 seem to be sufficient to cause the significant differences in branching patterns between teosinte and maize (Doebley et al., 1997). Maize was domesticated in Mesoamerica (Holst et al., 2007; Piperno et al., 2007; Pohl et al., 2007), while the other monocot crops belonging to the Panicoideae, pearl millet and sorghum, were selected in Sub-Saharan Africa (Remigereau et al., 2011). Interestingly, comparative QTL mapping revealed that also in pearl millet, TB1 was the molecular target of domestication (Remigereau et al., 2011). Polymorphism analyses comparing cultivated pearl millet with the wild form Pennisetum glaucum showed that the nucleotide diversity of the TB1 gene dramatically dropped in a region upstream of the transcription start site. This analysis indicates that nucleotide changes important for the reduced branching of pearl millet occurred within the promoter region of the TB1 gene (Remigereau et al., 2011). Such decreases of polymorphism restricted to single genes are characteristic of domestication events in contrast to evolutionary bottle necks that result in a reduction of polymorphism on the whole genome scale. Summarized, the studies in maize and pearl millet indicate that changes in the promoter activity and expression level of the domestication target gene TB1 may be causal for the reduced branching of some monocot crops.
To combine the knowledge on economic aspects of monocot crop architecture and to define targets of monocot crop breeding, an architectural ideotype that exhibits the ideal plant architecture (IPA) has been described (Lu et al., 2013). With respect to rice, this ideotype is characterized by low tiller number, high tiller productivity and a thick and strong culm (Jiao et al., 2010; Lu et al., 2013). Jiao et al. (2010) and Miura et al. (2010) both analyzed rice varieties that show IPA characteristics. Map based cloning attempts to isolate the quantitative trait loci that determine IPA resulted in the isolation of IPA1/OsSPL14, which is expressed at the shoot tip and in developing branches. This gene is negatively regulated by the microRNA OsmiR156. The low tillering Oryza japonica lines ST-12 and Shaoniejing that were independently analyzed by Jiao et al. (2010) and Miura et al. (2010), respectively, carry a mutation in the miR156 complementary site. Thus, in both lines, SPL14 mRNA is resistant to miR156-mediated degradation and accumulates to a higher RNA level than in the rice cultivars Nipponbare and Taichung Native 1 which were used as reference lines in map based cloning.
IPA1/OsSPL14 encodes the transcription factor SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 14. A DNA motif that is bound by IPA1/OsSPL14 was found in the OsTB1 promoter (Lu et al., 2013). The fact that a transgenic rice line that produces a miR156 resistant IPA1/SPL14 mRNA exhibits higher OsTB1 transcript levels indicates that IPA1/SPL14 positively regulates OsTB1 expression. As described above, TB1 is an important target during domestication and increased expression of OsTB1 leads to suppression of bud outgrowth, which most likely causes the observed low tillering phenotype of the analyzed rice lines with characteristics of IPA. However, low tillering is not the only characteristic of IPA. The O. japonica lines ST-12 and Shaoniejing also exhibit taller and stronger culms. This observation points to a pleiotropic action of IPA1/SPL14. In addition to bud outgrowth suppression caused by higher expression of OsTB1, increased plant height and higher grain number per panicle may be mediated by induction of DENSE AND ERECT PANICLE1 (DEP1; Huang et al., 2009) through IPA1/SPL14 (Lu et al., 2013). Besides from cultivars with an altered miR156 – IPA1/SPL14 pathway which were selected by classical breeding during crop domestication, a biotechnological approach, in which miR156 was overexpressed in switchgrass, was successful. The overexpressing lines exhibited increased tillering and also the biomass quantity and quality were improved, which is beneficial for the use of switchgrass as a resource of bioenergy (Fu et al., 2012).
Another example for tillering-relevant genes are STRIGOLACTONE BIOSYNTHESIS 1 and 2 (SLB1 and SLB2). Cardoso et al. (2014) identified these closely related genes by QTL mapping in rice. They are present in the low-tillering cultivar Azucena (Japonica subspecies), while they are absent from the high-tillering cultivar Bala (Indica subspecies) due to a genomic rearrangement. Both genes show high orthology to the Arabidopsis SL biosynthesis gene MAX1 and are functional in Arabidopsis, since they can rescue the max1 mutant phenotype (Cardoso et al., 2014). More recently, they were shown to catalyze the oxidation and subsequent hydroxylation of carlactone to yield the SL orobanchol (Zhang et al., 2014). Consistently, the cultivar Bala exudes low SL levels from roots (Cardoso et al., 2014). A generally reduced SL production would explain the high tillering phenotype and indicate that SLs are also important regulators of the architecture of crop plants, besides from the factors discussed above.
The initial reason for the QTL mapping, however, was not plant architecture. SLs are exuded by roots into the rhizosphere, where they promote arbuscular mycorrhiza, especially under phosphate starvation conditions. Root parasitic plants, such as Striga, appear to exploit this mechanism and use SLs as germination cues (reviewed in Bouwmeester et al., 2007). SLs therefore induce germination of Striga seeds, which is in line with the finding that the rice cultivar Azucena, exhibiting high SL exudation, is more susceptible to Striga infection (Cardoso et al., 2014). Therefore, SLs also play an important role in plant resistance in addition to their function in the control of rice tillering. Thus, they are a potent target for breeding efforts for improving agronomical traits in crop plants. Furthermore, a recent publication indicates that SL genes may also be a quantitative trait in trees used on short rotation plantations. The willow ortholog of MAX4 co-localizes with a QTL for shoot resprouting after coppicing (Salmon et al., 2014). Thus, manipulation of the SL pathway for improvement of crop plants may specifically be useful for fast growing trees like willow and poplar which are cultivated on short rotation coppices. These trees are grown for 3–5 years and, after harvesting, the plants are allowed to resprout from the stool to start the next rotation.
In addition to the degree of tillering, the angle between tiller and culm determines the suitability of rice varieties for rice farming (Wang and Li, 2005). Tillers of the wild rice Oryza rufipogon grow in a horizontal orientation during the vegetative phase. This horizontal growth habit suppresses competing weeds, but the horizontal tillers have high space requirements and are not suitable for cultivation of rice in dense stands. Thus, rice varieties with a more compact growth due to a smaller tiller angle were selected during domestication.
Yu et al. (2007) isolated TILLER ANGLE CONTROL1 (TAC1) by map-based cloning in an attempt to characterize a quantitative trait locus that decreases the tiller angle in rice. They used a mapping population obtained from a rice variety with almost zero tiller angle (straight tillers, compact growth) and a line with spread out tillers. The rice variety with compact growth carries a mutation in the 3′UTR of TAC1 which leads to aberrant splicing. The resulting mRNA contains a mutated 3′UTR that leads to decreased stability. Yu et al. (2007) could show that high levels of TAC1 mRNA correspond to a large tiller angle and low expression levels to a smaller tiller angle, respectively. Analysis of 152 rice accessions (wild type, O. japonica and O. indica cultivars) revealed that all lines with low tiller angle carry the identical tac1 mutation that leads to aberrant splicing of the tac1 transcript (Yu et al., 2007).
TAC1 shows sequence similarity to LAZY1, a gene that is also involved in tiller angle determination. In contrast to tac1, a loss of function in lazy1 results in wider tiller angles. This effect on tiller angle is caused by a modified gravitropic response of the mutant. In the lazy1 mutant, the apical-basal polar auxin transport is increased, while lateral auxin transport is decreased. This results in abnormal auxin distribution leading to a weaker gravitropic response. Therefore, LAZY1 controls gravitropism by regulating polar auxin transport (Li et al., 2007).
In conclusion, TAC1 and LAZY1 have opposite functions with respect to branch angle control (Figure 2B). The most obvious difference on the sequence level between TAC1 and LAZY1 is an EAR like domain at the C-terminus that is only present in LAZY1 (Dardick et al., 2013). Phylogenetic analyses and studies of intron–exon structure indicate that LAZY1 is, from an evolutionary perspective, the older gene and TAC1 evolved from LAZY1 (Dardick et al., 2013). The opposing activities of these transcription factors may be explained by affinity of TAC1 and LAZY1 for the same promoter motifs. LAZY1 likely acts as a repressor through the EAR domain. TAC1, which lacks the EAR domain, may compete with LAZY1 and diminish repression by LAZY1 (Dardick et al., 2013).
Other genes that regulate tiller angle are PROSTRATE GROWTH1 (PROG1; Tan et al., 2008) and LOOSE PLANT ARCHITECTURE 1 (LPA1; Wu et al., 2013). Both genes encode putative zinc finger transcription factors with C-terminal EAR-like repression domains. The tiller base in the prog1 mutant shows asymmetric growth due to a higher cell number on the lower side of the tiller base. Like lazy1, the lpa1 mutant exhibits reduced shoot gravitropism, possibly caused by a slower sedimentation of amyloplasts in the statocytes (Wu et al., 2013).
In summary, the analyses on TB1, TAC1, LAZY1, PROG1, and LPA1 in crop plants indicate that with respect to plant architecture, only few key genes have been the target of selection during domestication.
In the studies mentioned above, monocots were investigated. However, TAC1 has also been identified as a candidate gene for branch angle control in dicotyledonous species, e.g., in peach trees (Prunus persica; Dardick et al., 2013). In trees, fruit and wood production are influenced by crown architecture. Trees with compact crowns are suited for high density cultivation and allow yield increases compared to lines with a wider crown (Dardick et al., 2013). P. persica varieties that exhibit a compact growth habit are called broomy or pillar lines and the associated semidominant mutation has been designated as br. The mutation was mapped as an insertion that introduces a premature stop codon in a gene encoding a protein with similarity to the monocot TAC1. A knockout of the orthologous gene in Arabidopsis resulted in smaller angles between cauline (i.e., inflorescence) branches and the main inflorescence shoot as well as between rosette branches and the stem. The pyramid poplar (Populus nigra ‘Italica’) develops a phenotype comparable to the broomy or pillar variety of peach. This poplar growth habit may also be caused by a defect in a poplar ortholog of TAC1. In apple, another compact growth phenotype exists which has been designated columnar (co). However, this phenotype is different from the P. persica broomy or pillar growth habit. Columnar apple is not only characterized by a compact crown, but also by shorter branches, a thicker stem with shorter internodes and short fruit spurs (Petersen and Krost, 2013). Moreover, the br mutation is semidominant, while co is dominant. The co mutation has been mapped to a region of 393 kb with 36 ORFs on chromosome 10 (Petersen and Krost, 2013). However, the exact locus and its molecular function remain to be determined.
In fruit and timber trees, not only the branch angle, but also the degree of branching is economically important. The leaves of branches contribute to the specific leaf area index which significantly affects photosynthesis rate (Broeckx et al., 2012). In contrast to annual plants, trees build two different types of branches. During the growth period, the shoot apex suppresses the outgrowth of buds to a certain extent (apical dominance), leading to so-called paradormancy. However, this state of dormancy can be overcome by several factors (e.g., by decapitation), leading to bud outgrowth. Buds that develop and grow out in the same season without an intervening dormant season form so-called sylleptic branches. However, many species in temperate regions undergo dormancy during winter as an adaptation to adverse environmental conditions. After the growth period in summer, short day length and low temperatures prohibit further growth. The resulting stage of dormancy is called ecodormancy. It can still be broken if the growth conditions become more favorable. However, after further exposure to short daylength and low temperatures, the tree enters a stage called endodormancy, in which it can survive the harsh conditions in winter. Endodormancy can only be broken after a certain chilling requirement, i.e., a certain cumulative time of cold temperatures, is fulfilled. The plant is then reverted into an ecodormancy state, which will be broken when the environmental conditions become more favorable in spring. Buds formed during the previous growth period will then grow out and produce so-called proleptic branches. The different stages of dormancy described above are reviewed in Allona et al. (2008). Many tree species of the temperate regions form exclusively proleptic branches, but some genera like Populus, Prunus, Alnus, Larix, and Tsuga can also grow sylleptic branches (Broeckx et al., 2012). This may be advantageous during the establishment phase of trees since all branches that are built during the first growth period are, by definition, sylleptic branches. The additional leaf area of sylleptic branches contributes to carbon fixation and sylleptic branches have a high translocation efficiency of photosynthates (Scarascia-Mugnozza et al., 1999). Early canopy closure and the resulting suppression of weed growth might also be an important trait for fast growing trees on short rotation plantations.
In perennial plants, apical dominance seems to be controlled in a similar way as in annuals. Studies by Cline and Dong-Il (2002) indicate that auxin is a key player in this process. They compared three poplar clones with significant differences in sylleptic branching. They showed that “branchiness” of the three poplar clones correlates with sensitivity to auxin, which generally suppresses bud outgrowth: the clone with a low degree of sylleptic branching was more sensitive to auxin than the highly branched clone. A hallmark of branching control by apical dominance is a gradient of bud outgrowth across the main shoot. This is most evident and has been thoroughly characterized in Arabidopsis. Before flowering, bud initiation and outgrowth occurs in an acropetal direction while after the onset of flowering, this gradient is reversed and uppermost buds elongate and grow out first (Hempel and Feldman, 1994). Similarly, sylleptic branching occurs in a basipetal direction in poplar. In contrast, all proleptic branches that form after a period of dormancy start to elongate at a similar time point (Wilson, 2000). This synchronized growth of proleptic branches points to a control mechanism that is different from apical dominance or it is due to a factor that very efficiently breaks apical dominance. Studies by Moreno-Cortes et al. (2012) identified a protein that may play a role in bud outgrowth control. They isolated CsRAV1 from chestnut that encodes a protein with homology to AtRAV1 from Arabidopsis that has been classified as a transcriptional repressor (Ikeda and Ohme-Takagi, 2009). Overexpression of CsRAV1 in poplar induced a high degree of sylleptic branching. Since the poplar clone that was used in these studies usually does not branch during the first growth period (i.e., it does not form sylleptic branches), suppression of branching must have been released by constitutive overexpression of CsRAV1. Interestingly, CsRAV1 is highly expressed during winter. Moreno-Cortes et al. (2012) hypothesized that in perennials that grow in temperate regions, RAV1 accumulates during winter and elicits growth of proleptic branches from axillary meristems in the following spring. Overexpression of RAV1, thus, leads to season-independent accumulation of RAV1 and causes growth of sylleptic branches from meristems which have not been exposed to a period of winter dormancy.
Conclusion
Apical dominance as a key control mechanism of branching has been a focus of intense research since Thimann and Skoog performed experiments in the 1930s on the role of auxin in suppression of branching (Thimann and Skoog, 1933). As it became evident that auxin does not directly suppress bud outgrowth, the second messenger hypothesis was put forward and the search for the elusive branching hormones initiated. Cytokinin was soon classified as one of the second messengers (Turnbull et al., 1997; Müller and Leyser, 2011), but it took until 2008 to identify SL as another branching hormone (Gomez-Roldan et al., 2008; Umehara et al., 2008). Within 6 years after this discovery, canonical SL biosynthesis and signaling pathways were established (Waldie et al., 2014). Now, SLs are accepted as branching control factors for herbaceous monocots (Umehara et al., 2008) and dicots (Gomez-Roldan et al., 2008). Loss-of-function mutants of SL biosynthesis and signaling show profound changes of plant architecture. Nonetheless, modification of the SL pathway has not yet been used in genetic engineering to improve architecture of crop plants. Also, SL genes have not been a target during monocot domestication, since the architectural trait selected during domestication of crops is low branching (Doust, 2007). However, the identification of SLB1 and SLB2 in rice cultivars (see discussion above) points to a role of SLs in parasitic weed resistance. Therefore, SLs may be an important target in breeding programs.
Analyses of domestication genes in monocot crops led to the identification of a small set of target genes (Doebley et al., 1997; Doust, 2007; Yu et al., 2007; Tan et al., 2008; Ku et al., 2011; Remigereau et al., 2011), of which each profoundly influences plant architecture. Originally, researchers proposed that monocot genes controlling plant architecture, e.g., TB1 and TAC1, are unique to monocots (Doebley et al., 1997; Yu et al., 2007). However, orthologs of TB1 and TAC1 were soon also identified in dicots (Aguilar-Martinez et al., 2007; Martin-Trillo et al., 2011; Braun et al., 2012; Dardick et al., 2013). Now, with this knowledge, key genes for genetic engineering or for use as genetic markers for classical breeding of monocot and dicot crops are available.
In contrast to herbaceous plants, knowledge on branching control in woody plants generally is scarce. Currently, this topic is attracting more attention. Recent studies by Ward et al. (2013) and Czarnecki et al. (2014) showed complementation of Arabidopsis max mutants by Salix and Populus MAX orthologs, respectively, pointing to a role of SLs in trees. Also, PpeTAC1 has been characterized as a controlling factor of branch angle in peach (Dardick et al., 2013) and CsRAV1 from chestnut has been shown to play a role in seasonal control of proleptic branching (Moreno-Cortes et al., 2012).
Tree breeding is time consuming due to the long generation time of woody plants. Thus, using these key genes in genetic engineering approaches would be more straightforward to improve productivity. However, transgenic crops and also transgenic trees are not readily accepted by the public in many countries (Kaiser, 2001). Therefore, the generation of transgenic tree cultivars for wood or fruit production appears to be not economically reasonable at the moment. Alternatively, markers like broomy could be employed to assist classical breeding programs. Another powerful technique is Targeting Induced Local Lesions in Genomes (TILLING), which can identify desired point mutations in a mutagenized population in an efficient, high-throughput way (McCallum et al., 2000). A variant of this technique, called Ecotilling (Comai et al., 2004), could be used to screen natural populations for desired polymorphisms in order to exploit natural variation for breeding. These methods work without the production of genetically modified organisms (GMOs).
Additionally, targeted genome editing approaches such as CRISPR-Cas9 and related technologies may be used to actively introduce highly specific changes in the genome instead of screening for random changes (reviewed in Sander and Joung, 2014). However, it is still unclear how this and other new methods will be treated by legislature. Although, the resulting engineered plants cannot be distinguished from plants generated by traditional breeding methods, they may be classified as GMOs at least in the European Union, because their production involves transgenic intermediates (reviewed in Hartung and Schiemann, 2014).
Furthermore, even though the techniques discussed above are very powerful and may not fall under GMO-regulation, they are still limited to modifications of existing sequence within a given species. The introduction of entirely new sequences, allowing the attainment of completely new traits, can only be achieved by introducing foreign DNA, inevitably resulting in GMO by definition. Therefore, transgenic plants are still not entirely dispensable to match the demand for efficient crops and will most likely play a major role in the future in many countries.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We acknowledge funding by the German Ministry of Education and Research (FKZ 0315972C).
References
Abe, S., Sado, A., Tanaka, K., Kisugi, T., Asami, K., Ota, S., et al. (2014). Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. U.S.A. 111, 18084–18089. doi: 10.1073/pnas.1410801111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alder, A., Jamil, M., Marzorati, M., Bruno, M., Vermathen, M., Bigler, P., et al. (2012). The Path from beta-carotene to carlactone, a strigolactone-like plant hormone. Science 335, 1348–1351. doi: 10.1126/science.1218094
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Allona, I., Ramos, A., Ibanez, C., Contreras, A., Casado, R., and Aragoncillo, C. (2008). Molecular control of winter dormancy establishment in trees. Span. J. Agric. Res. 6, 201–210. doi: 10.5424/sjar/200806S1-389
CrossRef Full Text | Google Scholar
Arite, T., Iwata, H., Ohshima, K., Maekawa, M., Nakajima, M., Kojima, M., et al. (2007). DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J. 51, 1019–1029. doi: 10.1111/j.1365-313X.2007.03210.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Balla, J., Kalousek, P., Reinoehl, V., Friml, J., and Prochazka, S. (2011). Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J. 65, 571–577. doi: 10.1111/j.1365-313X.2010.04443.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bangerth, F. (1994). Response of cytokinin concentration in the xylem exudate of bean (Phaseolus vulgaris L) plants to decapitation and auxin treatment, and relation to apical dominance. Planta 194, 439–442. doi: 10.1007/BF00197546
CrossRef Full Text | Google Scholar
Barker, N. P., Clark, L. G., Davis, J. I., Duvall, M. R., Guala, G. F., Hsiao, C., et al. (2001). Phylogeny and subfamilial classification of the grasses (Poaceae). Ann. Mo. Bot. Gard. 88, 373–457. doi: 10.2307/3298585
CrossRef Full Text | Google Scholar
Bell, E. M., Lin, W.-C., Husbands, A. Y., Yu, L., Jaganatha, V., Jablonska, B., et al. (2012). Arabidopsis lateral organ boundaries negatively regulates brassinosteroid accumulation to limit growth in organ boundaries. Proc. Natl. Acad. Sci. U.S.A. 109, 21146–21151. doi: 10.1073/pnas.1210789109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bennett, T., Sieberer, T., Willett, B., Booker, J., Luschnig, C., and Leyser, O. (2006). The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 16, 553–563. doi: 10.1016/j.cub.2006.01.058
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beveridge, C. A., Symons, G. M., Murfet, I. C., Ross, J. J., and Rameau, C. (1997). The rms1 mutant of pea has elevated indole-3-acetic acid levels and reduced root-sap zeatin riboside content but increased branching controlled by graft-transmissible signal(s). Plant Physiol. 115, 1251–1258.
Google Scholar
Beveridge, C. A., Symons, G. M., and Turnbull, C. G. N. (2000). Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes RMS1 and RMS2. Plant Physiol. 123, 689–697. doi: 10.1104/pp.123.2.689
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Booker, J., Auldridge, M., Wills, S., Mccarty, D., Klee, H., and Leyser, O. (2004). MAX3/CCD7 is a carotenoid cleavage dioxygenase required for the synthesis of a novel plant signaling molecule. Curr. Biol. 14, 1232–1238. doi: 10.1016/j.cub.2004.06.061
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Booker, J., Sieberer, T., Wright, W., Williamson, L., Willett, B., Stirnberg, P., et al. (2005). MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell 8, 443–449. doi: 10.1016/j.devcel.2005.01.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Braun, N., De Saint Germain, A., Pillot, J.-P., Boutet-Mercey, S., Dalmais, M., Antoniadi, I., et al. (2012). The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 158, 225–238. doi: 10.1104/pp.111.182725
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brewer, P. B., Dun, E. A., Ferguson, B. J., Rameau, C., and Beveridge, C. A. (2009). Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 150, 482–493. doi: 10.1104/pp.108.134783
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Broeckx, L. S., Verlinden, M. S., Vangronsveld, J., and Ceulemans, R. (2012). Importance of crown architecture for leaf area index of different Populus genotypes in a high-density plantation. Tree Physiol. 32, 1214–1226. doi: 10.1093/treephys/tps083
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Busch, B. L., Schmitz, G., Rossmann, S., Piron, F., Ding, J., Bendahmane, A., et al. (2011). Shoot branching and leaf dissection in tomato are regulated by homologous gene modules. Plant Cell 23, 3595–3609. doi: 10.1105/tpc.111.087981
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cardoso, C., Zhang, Y., Jamil, M., Hepworth, J., Charnikhova, T., Dimkpa, S. O. N., et al. (2014). Natural variation of rice strigolactone biosynthesis is associated with the deletion of two MAX1 orthologs. Proc. Natl. Acad. Sci. U.S.A. 111, 2379–2384. doi: 10.1073/pnas.1317360111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Casal, J. J., Sanchez, R. A., and Deregibus, V. A. (1986). The effect pf plant-density on tillering – the involvement of R/FR ratio and the proportion of radiation intercepted per plant. Environ. Exp. Bot. 26, 365–371. doi: 10.1016/0098-8472(86)90024-9
CrossRef Full Text | Google Scholar
Cline, M. G., and Dong-Il, K. (2002). A preliminary investigation of the role of auxin and cytokinin in sylleptic branching of three hybrid poplar clones exhibiting contrasting degrees of sylleptic branching. Ann. Bot. 90, 417–421. doi: 10.1093/aob/mcf195
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Comai, L., Young, K., Till, B. J., Reynolds, S. H., Greene, E. A., Codomo, C. A., et al. (2004). Efficient discovery of DNA polymorphisms in natural populations by Ecotilling. Plant J. 37, 778–786. doi: 10.1111/j.0960-7412.2003.01999.x
CrossRef Full Text | Google Scholar
Czarnecki, O., Yang, J., Wang, X., Wang, S., Muchero, W., Tuskan, G. A., et al. (2014). Characterization of MORE AXILLARY GROWTH genes in Populus. PLoS ONE 9:e102757. doi: 10.1371/journal.pone.0102757
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dardick, C., Callahan, A., Horn, R., Ruiz, K. B., Zhebentyayeva, T., Hollender, C., et al. (2013). PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family found in diverse plants species. Plant J. 75, 618–630. doi: 10.1111/tpj.12234
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
de Jong, M., George, G., Ongaro, V., Williamson, L., Willetts, B., Ljung, K., et al. (2014). Auxin and strigolactone signaling are required for modulation of Arabidopsis shoot branching by nitrogen supply. Plant Physiol. 166, 384–395. doi: 10.1104/pp.114.242388
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drummond, R. S. M., Sheehan, H., Simons, J. L., Martinez-Sanchez, N. M., Turner, R. M., Putterill, J., et al. (2012). The expression of petunia strigolactone pathway genes is altered as part of the endogenous developmental program. Front. Plant Sci. 2:115. doi: 10.3389/fpls.2011.00115
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Emery, J. F., Floyd, S. K., Alvarez, J., Eshed, Y., Hawker, N. P., Izhaki, A., et al. (2003). Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr. Biol. 13, 1768–1774. doi: 10.1016/j.cub.2003.09.035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Euring, D., Loefke, C., Teichmann, T., and Polle, A. (2012). Nitrogen fertilization has differential effects on N allocation and lignin in two Populus species with contrasting ecology. Trees 26, 1933–1942. doi: 10.1007/s00468-012-0761-0
CrossRef Full Text | Google Scholar
Everat-Bourbouloux, A., and Bonnemain, J. L. (1980). Distribution of labeled auxin and derivatives in stem tissues of intact and decapitated broad-bean plants in relation to apical dominance. Physiol. Plant. 50, 145–152. doi: 10.1111/j.1399-3054.1980.tb04441.x
CrossRef Full Text | Google Scholar
Faiss, M., Zalubilova, J., Strnad, M., and Schmülling, T. (1997). Conditional transgenic expression of the ipt gene indicates a function for cytokinins in paracrine signaling in whole tobacco plants. Plant J. 12, 401–415. doi: 10.1046/j.1365-313X.1997.12020401.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Foo, E., Buillier, E., Goussot, M., Foucher, F., Rameau, C., and Beveridge, C. A. (2005). The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17, 464–474. doi: 10.1105/tpc.104.026716
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Foo, E., Yoneyama, K., Hugill, C. J., Quittenden, L. J., and Reid, J. B. (2013). Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant. 6, 76–87. doi: 10.1093/mp/sss115
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Forde, B., and Lorenzo, H. (2001). The nutritional control of root development. Plant Soil 232, 51–68. doi: 10.1023/A:1010329902165
CrossRef Full Text | Google Scholar
Franzke, A., Lysak, M. A., Al-Shehbaz, I. A., Koch, M. A., and Mummenhoff, K. (2011). Cabbage family affairs: the evolutionary history of Brassicaceae. Trends Plant Sci. 16, 108–116. doi: 10.1016/j.tplants.2010.11.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fu, C., Sunkar, R., Zhou, C., Shen, H., Zhang, J.-Y., Matts, J., et al. (2012). Overexpression of miR156 in switchgrass (Panicum virgatum L.) results in various morphological alterations and leads to improved biomass production. Plant Biotech. J. 10, 443–452. doi: 10.1111/j.1467-7652.2011.00677.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Furutani, M., Vernoux, T., Traas, J., Kato, T., Tasaka, M., and Aida, M. (2004). PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131, 5021–5030. doi: 10.1242/dev.01388
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gallavotti, A., Zhao, Q., Kyozuka, J., Meeley, R. B., Ritter, M., Doebley, J. F., et al. (2004). The role of barren stalk1 in the architecture of maize. Nature 432, 630–635. doi: 10.1038/nature03148
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gendron, J. M., Liu, J.-S., Fan, M., Bai, M.-Y., Wenkel, S., Springer, P. S., et al. (2012). Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis. Proc. Natl. Acad. Sci. U.S.A. 109, 21152–21157. doi: 10.1073/pnas.1210799110
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gomez-Roldan, V., Fermas, S., Brewer, P. B., Puech-Pages, V., Dun, E. A., Pillot, J.-P., et al. (2008). Strigolactone inhibition of shoot branching. Nature 455, 189–194. doi: 10.1038/nature07271
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grbic, V., and Bleecker, A. B. (2000). Axillary meristem development in Arabidopsis thaliana. Plant J. 21, 215–223. doi: 10.1046/j.1365-313x.2000.00670.x
CrossRef Full Text | Google Scholar
Greb, T., Clarenz, O., Schafer, E., Muller, D., Herrero, R., Schmitz, G., et al. (2003). Molecular analysis of the LATERAL SUPPRESSOR gene in Arabidopsis reveals a conserved control mechanism for axillary meristem formation. Gene Dev. 17, 1175–1187. doi: 10.1101/gad.260703
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Guo, S., Xu, Y., Liu, H., Mao, Z., Zhang, C., Ma, Y., et al. (2013). The interaction between OsMADS57 and OsTB1 modulates rice tillering via DWARF14. Nat. Commun. 4, 1566. doi: 10.1038/ncomms2542
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hagen, G., and Guilfoyle, T. (2002). Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 49, 373–385. doi: 10.1023/A:1015207114117
CrossRef Full Text | Google Scholar
Hamiaux, C., Drummond, R. S. M., Janssen, B. J., Ledger, S. E., Cooney, J. M., Newcomb, R. D., et al. (2012). DAD2 is an α/β hydrolase likely to be involved in the perception of the plant branching hormone, strigolactone. Curr. Biol. 22, 2032–2036. doi: 10.1016/j.cub.2012.08.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hempel, F. D., and Feldman, L. J. (1994). Biderectional inflorescence development in Arabidopsis thaliana-acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192, 276–286. doi: 10.1007/BF01089045
CrossRef Full Text | Google Scholar
Holst, I., Moreno, J. E., and Piperno, D. R. (2007). Identification of teosinte, maize, and Tripsacum in Mesoamerica by using pollen, starch grains, and phytoliths. Proc. Natl. Acad. Sci. U.S.A. 104, 17608–17613. doi: 10.1073/pnas.0708736104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Huang, X., Qian, Q., Liu, Z., Sun, H., He, S., Luo, D., et al. (2009). Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497. doi: 10.1038/ng.352
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hunter, P. J., Teakle, G. R., and Bending, G. D. (2014). Root traits and microbial community interactions in relation to phosphorus availability and acquisition, with particular reference to Brassica. Front. Plant Sci. 5:27. doi: 10.3389/fpls.2014.00027
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ikezaki, M., Kojima, M., Sakakibara, H., Kojima, S., Ueno, Y., Machida, C., et al. (2010). Genetic networks regulated by ASYMMETRIC LEAVES1 (AS1) and AS2 in leaf development in Arabidopsis thaliana: KNOX genes control five morphological events. Plant J. 61, 70–82. doi: 10.1111/j.1365-313X.2009.04033.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ishikawa, S., Maekawa, M., Arite, T., Onishi, K., Takamure, I., and Kyozuka, J. (2005). Suppression of tiller bud activity in tillering dwarf mutants of rice. Plant Cell Physiol. 46, 79–86. doi: 10.1093/pcp/pci022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jiang, L., Liu, X., Xiong, G., Liu, H., Chen, F., Wang, L., et al. (2013). DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–407. doi: 10.1038/nature12870
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jiao, Y., Wang, Y., Xue, D., Wang, J., Yan, M., Liu, G., et al. (2010). Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544. doi: 10.1038/ng.591
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johnson, X., Brcich, T., Dun, E. A., Goussot, M., Haurogne, K., Beveridge, C. A., et al. (2006). Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 142, 1014–1026. doi: 10.1104/pp.106.087676
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kagiyama, M., Hirano, Y., Mori, T., Kim, S.-Y., Kyozuka, J., Seto, Y., et al. (2013). Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells 18, 147–160. doi: 10.1111/gtc.12025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kalousek, P., Buchtova, D., Balla, J., Reinohl, V., and Prochazk, S. (2010). Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ. Agric. Silvic. Mendelianae Brun. 58, 79–88. doi: 10.11118/actaun201058040079
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kebrom, T. H., Brutnell, T. P., and Finlayson, S. A. (2010). Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signalling pathways. Plant Cell Environ. 33, 48–58. doi: 10.1111/j.1365-3040.2009.02050.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kebrom, T. H., Burson, B. L., and Finlayson, S. A. (2006). Phytochrome B represses teosinte branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 140, 1109–1117. doi: 10.1104/pp.105.074856
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keller, T., Abbott, J., Moritz, T., and Doerner, P. (2006). Arabidopsis REGULATOR OF AXILLARY MERISTEMS1 controls a leaf axil stem cell niche and modulates vegetative development. Plant Cell 18, 598–611. doi: 10.1105/tpc.105.038588
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kohlen, W., Charnikhova, T., Liu, Q., Bours, R., Domagalska, M. A., Beguerie, S., et al. (2011). Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 155, 974–987. doi: 10.1104/pp.110.164640
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Komatsu, M., Maekawa, M., Shimamoto, K., and Kyozuka, J. (2001). The LAX1 and FRIZZY PANICLE 2 genes determine the inflorescence architecture of rice by controlling rachis-branch and spikelet development. Dev. Biol. 231, 364–373. doi: 10.1006/dbio.2000.9988
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Komatsu, K., Maekawa, M., Ujiie, S., Satake, Y., Furutani, I., Okamoto, H., et al. (2003). LAX and SPA: major regulators of shoot branching in rice. Proc. Natl. Acad. Sci. U.S.A. 100, 11765–11770. doi: 10.1073/pnas.1932414100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ku, L., Wei, X., Zhang, S., Zhang, J., Guo, S., and Chen, Y. (2011). Cloning and characterization of a putative TAC1 ortholog associated with leaf angle in maize (Zea mays L.). PLoS ONE 6:e20621. doi: 10.1371/journal.pone.0020621
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lewis, J. M., Mackintosh, C. A., Shin, S., Gilding, E., Kravchenko, S., Baldridge, G., et al. (2008). Overexpression of the maize teosinte branched1 gene in wheat suppresses tiller development. Plant Cell Rep. 27, 1217–1225. doi: 10.1007/s00299-008-0543-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, C. J., and Bangerth, F. (1999). Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol. Plant. 106, 415–420. doi: 10.1034/j.1399-3054.1999.106409.x
CrossRef Full Text | Google Scholar
Li, P., Wang, Y., Qian, Q., Fu, Z., Wang, M., Zeng, D., et al. (2007). LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 17, 402–410. doi: 10.1038/cr.2007.38
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Li, X. Y., Qian, Q., Fu, Z. M., Wang, Y. H., Xiong, G. S., Zeng, D. L., et al. (2003). Control of tillering in rice. Nature 422, 618–621. doi: 10.1038/nature01518
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lin, H., Wang, R., Qian, Q., Yan, M., Meng, X., Fu, Z., et al. (2009). DWARF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell 21, 1512–1525. doi: 10.1105/tpc.109.065987
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lopez-Bucio, J., Hernandez-Abreu, E., Sanchez-Calderon, L., Nieto-Jacobo, M. F., Simpson, J., and Herrera-Estrella, L. (2002). Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiol. 129, 244–256. doi: 10.1104/pp.010934
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lopez-Raez, J. A., Charnikhova, T., Gomez-Roldan, V., Matusova, R., Kohlen, W., De Vos, R., et al. (2008). Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 178, 863–874. doi: 10.1111/j.1469-8137.2008.02406.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lu, Z., Yu, H., Xiong, G., Wang, J., Jiao, Y., Liu, G., et al. (2013). Genome-wide binding analysis of the transcription activator ideal plant architecture1 reveals a complex network regulating rice plant architecture. Plant Cell 25, 3743–3759. doi: 10.1105/tpc.113.113639
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marhavy, P., Duclercq, J., Weller, B., Feraru, E., Bielach, A., Offringa, R., et al. (2014). Cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr. Biol. 24, 1031–1037. doi: 10.1016/j.cub.2014.04.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin-Trillo, M., Gonzalez Grandio, E., Serra, F., Marcel, F., Luisa Rodriguez-Buey, M., Schmitz, G., et al. (2011). Role of tomato BRANCHED1-like genes in the control of shoot branching. Plant J. 67, 701–714. doi: 10.1111/j.1365-313X.2011.04629.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mashiguchi, K., Sasaki, E., Shimada, Y., Nagae, M., Ueno, K., Nakano, T., et al. (2009). Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci. Biotech. Bioch. 73, 2460–2465. doi: 10.1271/bbb.90443
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mason, M. G., Ross, J. J., Babst, B. A., Wienclaw, B. N., and Beveridge, C. A. (2014). Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. U.S.A. 111, 6092–6097. doi: 10.1073/pnas.1322045111
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McCallum, C. M., Comai, L., Greene, E. A., and Henikoff, S. (2000). Targeting induced local lesions in genomes (TILLING) for plant functional genomics. Plant Physiol. 123, 439–442. doi: 10.1104/pp.123.2.439
CrossRef Full Text | Google Scholar
Minakuchi, K., Kameoka, H., Yasuno, N., Umehara, M., Luo, L., Kobayashi, K., et al. (2010). FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol. 51, 1127–1135. doi: 10.1093/pcp/pcq083
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miura, K., Ikeda, M., Matsubara, A., Song, X.-J., Ito, M., Asano, K., et al. (2010). OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 42, 545–549. doi: 10.1038/ng.592
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miyawaki, K., Matsumoto-Kitano, M., and Kakimoto, T. (2004). Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37, 128–138. doi: 10.1046/j.1365-313X.2003.01945.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moreno-Cortes, A., Hernandez-Verdeja, T., Sanchez-Jimenez, P., Gonzalez-Melendi, P., Aragoncillo, C., and Allona, I. (2012). CsRAV1 induces sylleptic branching in hybrid poplar. New Phytol. 194, 83–90. doi: 10.1111/j.1469-8137.2011.04023.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Morris, S. E., Cox, M. C. H., Ross, J. J., Krisantini, S., and Beveridge, C. A. (2005). Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 138, 1665–1672. doi: 10.1104/pp.104.058743
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nakamura, H., Xue, Y.-L., Miyakawa, T., Hou, F., Qin, H.-M., Fukui, K., et al. (2013). Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 4, 2613. doi: 10.1038/ncomms3613
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Niu, Y. F., Chai, R. S., Jin, G. L., Wang, H., Tang, C. X., and Zhang, Y. S. (2013). Responses of root architecture development to low phosphorus availability: a review. Ann. Bot. 112, 391–408. doi: 10.1093/aob/mcs285
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nordstrom, A., Tarkowski, P., Tarkowska, D., Norbaek, R., Astot, C., Dolezal, K., et al. (2004). Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc. Natl. Acad. Sci. U.S.A. 101, 8039–8044. doi: 10.1073/pnas.0402504101
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Otsuga, D., Deguzman, B., Prigge, M. J., Drews, G. N., and Clark, S. E. (2001). REVOLUTA regulates meristem initiation at lateral positions. Plant J. 25, 223–236. doi: 10.1046/j.1365-313x.2001.00959.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Paciorek, T., Zazimalova, E., Ruthardt, N., Petrasek, J., Stierhof, Y. D., Kleine-Vehn, J., et al. (2005). Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256. doi: 10.1038/nature03633
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peng, J. R., Richards, D. E., Hartley, N. M., Murphy, G. P., Devos, K. M., Flintham, J. E., et al. (1999). “Green revolution” genes encode mutant gibberellin response modulators. Nature 400, 256–261. doi: 10.1038/22307
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peret, B., Desnos, T., Jost, R., Kanno, S., Berkowitz, O., and Nussaume, L. (2014). Root architecture responses: in search of phosphate. Plant Physiol. 166, 1713–1723. doi: 10.1104/pp.114.244541
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Piperno, D. R., Moreno, J. E., Iriarte, J., Hoist, I., Lachniet, M., Jones, J. G., et al. (2007). Late pleistocene and holocene environmental history of the iguala valley, central balsas watershed of Mexico. Proc. Natl. Acad. Sci. U.S.A. 104, 11874–11881. doi: 10.1073/pnas.0703442104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pohl, M. E. D., Piperno, D. R., Pope, K. O., and Jones, J. G. (2007). Microfossil evidence for pre-Columbian maize dispersals in the neotropics from San Andres, Tabasco, Mexico. Proc. Natl. Acad. Sci. U.S.A. 104, 6870–6875. doi: 10.1073/pnas.0701425104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Prusinkiewicz, P., Crawford, S., Smith, R. S., Ljung, K., Bennett, T., Ongaro, V., et al. (2009). Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. U.S.A. 106, 17431–17436. doi: 10.1073/pnas.0906696106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rameau, C., Bertheloot, J., Leduc, N., Andrieu, B., Foucher, F., and Sakr, S. (2015). Multiple pathways regulate shoot branching. Front. Plant Sci. 5:714. doi: 10.3389/fpls.2014.00741
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Remigereau, M.-S., Lakis, G., Rekima, S., Leveugle, M., Fontaine, M. C., Langin, T., et al. (2011). Cereal domestication and evolution of branching: evidence for soft selection in the TB1 orthologue of pearl millet (Pennisetum glaucum L. R. Br.). PLoS ONE 6:e22404. doi: 10.1371/journal.pone.0022404
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sachs, T. (1981). The control of the patterned differentiation of vascular tissues. Adv. Bot. Res. 9, 151–262. doi: 10.1016/S0065-2296(08)60351-1
CrossRef Full Text | Google Scholar
Sachs, T., and Thimann, K. V. (1964). Release of lateral buds from apical dominance. Nature 201, 939–940. doi: 10.1038/201939a0
CrossRef Full Text | Google Scholar
Salmon, J., Ward, S. P., Hanley, S. J., Leyser, O., and Karp, A. (2014). Functional screening of willow alleles in Arabidopsis combined with QTL mapping in willow (Salix) identifies SxMAX4 as a coppicing response gene. Plant Biotech. J. 12, 480–491. doi: 10.1111/pbi.12154
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sasaki, A., Ashikari, M., Ueguchi-Tanaka, M., Itoh, H., Nishimura, A., Swapan, D., et al. (2002). Green revolution: a mutant gibberellin-synthesis gene in rice — new insight into the rice variant that helped to avert famine over thirty years ago. Nature 416, 701–702. doi: 10.1038/416701a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sauer, M., Balla, J., Luschnig, C., Wisniewska, J., Reinohl, V., Friml, J., et al. (2006). Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Gene Dev. 20, 2902–2911. doi: 10.1101/gad.390806
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scarascia-Mugnozza, G. E., Hinckley, T. M., Stettler, R. F., Heilman, P. E., and Isebrands, J. G. (1999). Production physiology and morphology of Populus species and their hybrids grown under short rotation. III. Seasonal carbon allocation patterns from branches. Can. J. For. Res. 29, 1419–1432. doi: 10.1139/cjfr-29-9-1419
CrossRef Full Text | Google Scholar
Schmitz, G., Tillmann, E., Carriero, F., Fiore, C., Cellini, F., and Theres, K. (2002). The tomato blind gene encodes a MYB transcription factor that controls the formation of lateral meristems. Proc. Natl. Acad. Sci. U.S.A. 99, 1064–1069. doi: 10.1073/pnas.022516199
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schumacher, K., Schmitt, T., Rossberg, M., Schmitz, C., and Theres, K. (1999). The lateral suppressor (Ls) gene of tomato encodes a new member of the VHIID protein family. Proc. Natl. Acad. Sci. U.S.A. 96, 290–295. doi: 10.1073/pnas.96.1.290
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Snow, R. (1925). The correlative inhibition of the growth of the axillary buds. Ann. Bot. 39, 841–859.
Google Scholar
Snowden, K. C., Simkin, A. J., Janssen, B. J., Templeton, K. R., Loucas, H. M., Simons, J. L., et al. (2005). The decreased apical dominance1/Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE8 gene affects branch production and plays a role in leaf senescence, root growth, and flower development. Plant Cell 17, 746–759. doi: 10.1105/tpc.104.027714
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sorefan, K., Booker, J., Haurogne, K., Goussot, M., Bainbridge, K., Foo, E., et al. (2003). MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 17, 1469–1474. doi: 10.1101/gad.256603
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sorokin, H. P., and Thimann, K. V. (1964). Histological basis for inhibition of axillary buds in Pisum sativum and effects of auxin and kinetin on xylem development. Protoplasma 59, 326–350. doi: 10.1007/BF01248566
CrossRef Full Text | Google Scholar
Spinelli, S. V., Paula Martin, A., Viola, I. L., Gonzalez, D. H., and Palatnik, J. F. (2011). A mechanistic link between STM and CUC1 during Arabidopsis development. Plant Physiol. 156, 1894–1904. doi: 10.1104/pp.111.177709
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stanga, J. P., Smith, S. M., Briggs, W. R., and Nelson, D. C. (2013). SUPPRESSOR OF MORE AXILLARY GROWTH2 1 controls seed germination and seedling development in Arabidopsis. Plant Physiol. 163, 318–330. doi: 10.1104/pp.113.221259
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stirnberg, P., Furner, I. J., and Ottoline Leyser, H. M. (2007). MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 50, 80–94. doi: 10.1111/j.1365-313X.2007.03032.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stirnberg, P., Liu, J.-P., Ward, S., Kendall, S. L., and Leyser, O. (2012a). Mutation of the cytosolic ribosomal protein-encoding RPS10B gene affects shoot meristematic function in Arabidopsis. BMC Plant Biol. 12:160. doi: 10.1186/1471-2229-12-160
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stirnberg, P., Zhao, S., Williamson, L., Ward, S., and Leyser, O. (2012b). FHY3 promotes shoot branching and stress tolerance in Arabidopsis in an AXR1-dependent manner. Plant J. 71, 907–920. doi: 10.1111/j.1365-313X.2012.05038.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stirnberg, P., Van De Sande, K., and Leyser, H. M. (2002). MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 129, 1131–1141.
PubMed Abstract | Full Text | Google Scholar
Sussex, I. M., and Kerk, N. M. (2001). The evolution of plant architecture. Curr. Opin. Plant Biol. 4, 33–37. doi: 10.1016/S1369-5266(00)00132-1
CrossRef Full Text | Google Scholar
Takeda, T., Suwa, Y., Suzuki, M., Kitano, H., Ueguchi-Tanaka, M., Ashikari, M., et al. (2003). The OsTB1 gene negatively regulates lateral branching in rice. Plant J. 33, 513–520. doi: 10.1046/j.1365-313X.2003.01648.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tan, L., Li, X., Liu, F., Sun, X., Li, C., Zhu, Z., et al. (2008). Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 40, 1360–1364. doi: 10.1038/ng.197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanaka, M., Takei, K., Kojima, M., Sakakibara, H., and Mori, H. (2006). Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J. 45, 1028–1036. doi: 10.1111/j.1365-313X.2006.02656.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Teo, Z. W. N., Song, S., Wang, Y.-Q., Liu, J., and Yu, H. (2014). New insights into the regulation of inflorescence architecture. Trends Plant Sci. 19, 158–165. doi: 10.1016/j.tplants.2013.11.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thimann, K. V., and Skoog, F. (1933). Studies on the growth hormone of plants: III. The inhibiting action of the growth substance on bud development. Proc. Natl. Acad. Sci. U.S.A. 19, 714–716. doi: 10.1073/pnas.19.7.714
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Turnbull, C. G. N., Raymond, M. A. A., Dodd, I. C., and Morris, S. E. (1997). Rapid increases in cytokinin concentration in lateral buds of chickpea (Cicer arietinum L) during release of apical dominance. Planta 202, 271–276. doi: 10.1007/s004250050128
CrossRef Full Text | Google Scholar
Umehara, M., Hanada, A., Magome, H., Takeda-Kamiya, N., and Yamaguchi, S. (2010). Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol. 51, 1118–1126. doi: 10.1093/pcp/pcq084
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Umehara, M., Hanada, A., Yoshida, S., Akiyama, K., Arite, T., Takeda-Kamiya, N., et al. (2008). Inhibition of shoot branching by new terpenoid plant hormones. Nature 455, 195–200. doi: 10.1038/nature07272
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vanneste, K., Maere, S., and Van De Peer, Y. (2014). Tangled up in two: a burst of genome duplications at the end of the Cretaceous and the consequences for plant evolution. Philos. Trans. R. Soc. Lon. B Sci. 369, 20130353. doi: 10.1098/rstb.2013.0353
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Q., Kohlen, W., Rossmann, S., Vernoux, T., and Theres, K. (2014a). Auxin depletion from the leaf axil conditions competence for axillary meristem formation in Arabidopsis and tomato. Plant Cell 26, 2068–2079. doi: 10.1105/tpc.114.123059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Y., Wang, J., Shi, B., Yu, T., Qi, J., Meyerowitz, E. M., et al. (2014b). The stem cell niche in leaf axils is established by auxin and cytokinin in Arabidopsis. Plant Cell 26, 2055–2067. doi: 10.1105/tpc.114.123083
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, Y., Sun, S., Zhu, W., Jia, K., Yang, H., and Wang, X. (2013). Strigolactone/MAX2-induced degradation of brassinosteroid transcriptional effector BES1 regulates shoot branching. Dev. Cell 27, 681–688. doi: 10.1016/j.devcel.2013.11.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ward, S. P., Salmon, J., Hanley, S. J., Karp, A., and Leyser, O. (2013). Using Arabidopsis to study shoot branching in biomass willow. Plant Physiol. 162, 800–811. doi: 10.1104/pp.113.218461
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Waters, M. T., Brewer, P. B., Bussell, J. D., Smith, S. M., and Beveridge, C. A. (2012). The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 159, 1073–1085. doi: 10.1104/pp.112.196253
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wenzel, C. L., Schuetz, M., Yu, Q., and Mattsson, J. (2007). Dynamics of MONOPTEROS and PIN-FORMED1 expression during leaf vein pattern formation in Arabidopsis thaliana. Plant J. 49, 387–398. doi: 10.1111/j.1365-313X.2006.02977.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Whipple, C. J., Kebrom, T. H., Weber, A. L., Yang, F., Hall, D., Meeley, R., et al. (2011). GRASSY TILLERS1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc. Natl. Acad. Sci. U.S.A. 108, E506–E512. doi: 10.1073/pnas.1102819108
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wu, X., Tang, D., Li, M., Wang, K., and Cheng, Z. (2013). Loose plant architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 161, 317–329. doi: 10.1104/pp.112.208496
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang, F., Wang, Q., Schmitz, G., Mueller, D., and Theres, K. (2012). The bHLH protein ROX acts in concert with RAX1 and LAS to modulate axillary meristem formation in Arabidopsis. Plant J. 71, 61–70. doi: 10.1111/j.1365-313X.2012.04970.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoneyama, K., Xie, X., Kisugi, T., Nomura, T., and Yoneyama, K. (2013). Nitrogen and phosphorus fertilization negatively affects strigolactone production and exudation in sorghum. Planta 238, 885–894. doi: 10.1007/s00425-013-1943-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoneyama, K., Xie, X., Kusumoto, D., Sekimoto, H., Sugimoto, Y., Takeuchi, Y., et al. (2007). Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta 227, 125–132. doi: 10.1007/s00425-007-0600-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yu, B., Lin, Z., Li, H., Li, X., Li, J., Wang, Y., et al. (2007). TAC1, a major quantitative trait locus controlling tiller angle in rice. Plant J. 52, 891–898. doi: 10.1111/j.1365-313X.2007.03284.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y., Van Dijk, A. D. J., Scaffidi, A., Flematti, G. R., Hofmann, M., Charnikhova, T., et al. (2014). Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 10, 1028–1033. doi: 10.1038/nchembio.1660
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhou, F., Lin, Q., Zhu, L., Ren, Y., Zhou, K., Shabek, N., et al. (2013). D14-SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature 504, 406–410. doi: 10.1038/nature12878
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar