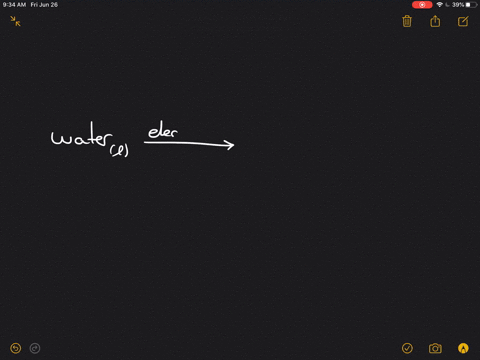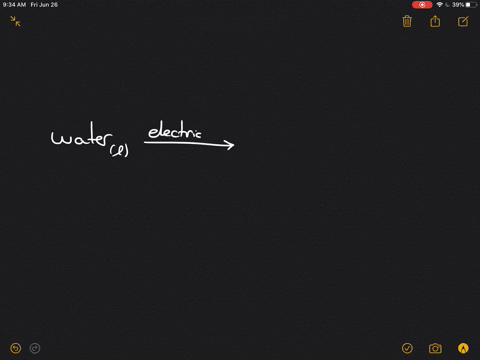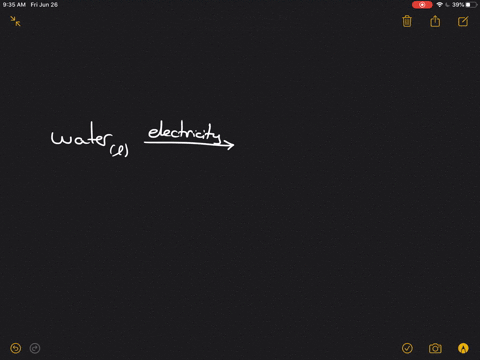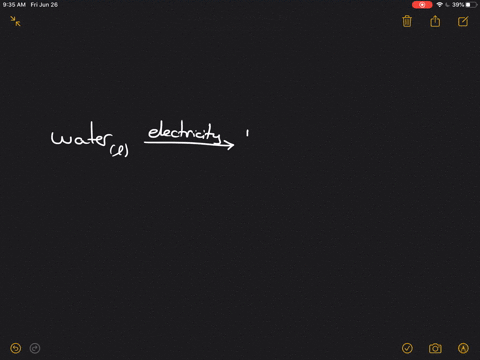In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
There are some key words to watch for when reading or writing a word equation. The words «and» or «plus» mean one chemical and another are both reactants or products. The phrase «is reacted with» indicates the chemicals are reactants. If you say «forms», «makes», or «yields», it means the following substances are products.
When you write a chemical equation from a word equation, the reactants always go on the lefthand side of the equation, while the reactants are on the righthand side. This is true even if the products are listed before the reactants in the word equation.
Key Takeaways: Word Equations
- A word equation is an expression of a chemical reaction or mathematical equation using words rather than letters, numbers, and operators.
- In chemistry, a word equation indicates the order of events of a chemical reaction. The number of moles and types of reactants yield the number of moles and types of products.
- Word equations help in learning chemistry because they reinforce the thought process involved in writing a chemical reaction or equation.
Word Equation Examples
The chemical reaction 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as:
hydrogen gas + oxygen gas → steam
As a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen.»
While a word equation doesn’t ordinarily include numbers or symbols (Example: You wouldn’t say «Two H two and one O two makes two H two O», sometimes it is necessary to use a number to indicate the oxidation state of a reactant so that a person writing a chemical equation can do it correctly. This is mostly for the transition metals, which can have multiple oxidation states.
For example, in the reaction between copper and oxygen to form copper oxide, the chemical formula of copper oxide and the number of copper and oxygen atoms involved depends on whether copper(I) or copper(II) participates in the reaction. In this case, it would be fine to say:
copper + oxygen → copper(II) oxide
or
Copper reacts with oxygen to produce copper two oxide.
The (unbalanced) chemical equation for the reaction would start out as:
Cu + O2 → CuO
Balancing the the equation yields:
2Cu + O2 → 2CuO
You would get a different equation and product formula using copper(I):
Cu + O2 → Cu2O
4Cu + O2 → 2Cu2O
More examples of word reactions include:
- Chlorine gas reacts with methane and carbon tetrachloride to produce hydrogen chloride.
- Adding sodium oxide to water produces sodium hydroxide.
- Iodine crystals and chlorine gas react to make solid iron and carbon dioxide gas.
- Zinc and lead two nitrate make zinc nitrate and lead metal.
which means: Zn + Pb (NO3)2 → Zn(NO3)2 + Pb
Why Use Word Equations?
When you’re learning general chemistry, work equations are used to help introduce the concepts of reactants, products, the direction of reactions, and to help you understand precision of language. They may seem annoying, but are a good introduction to the thought processes required for chemistry courses. In any chemical reaction, you need to be able to identify the chemical species that react with each other and what they make.
Word Equations in Other Sciences
Chemistry isn’t the only science to use equations. Physics equations and mathematical equations may also be expressed in words. Usually in these equations two statements are set to be equal to each other. For example, if you way «force equals mass multiplied by acceleration» then you are providing the word equation for the formula F = m*a. Other times, one side of the equation may be less than (<), greater than (>), less than or equal to, or greater than or equal to the other side of the equation. Addition, subtraction, multiplication, division, logs, square roots, integrals, and other operations can be stated in word equations. However, complex equations that contain parentheses to describe the order of operations are very hard to understand as word equations.
Source
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (December 14, 2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
If you need to use an equation, add or write it in Word. Select Insert > Equation or press Alt + =. To use a built-in formula, select Design > Equation. To create your own, select Design > Equation > Ink Equation.
Contents
- 1 What is a word equation give an example?
- 2 What is a word equation and how it is written?
- 3 What is simple equation?
- 4 What is word equation and how is it written class 10?
- 5 What is the word equation for sodium and water?
- 6 How do you write an equation for a reaction?
- 7 How many words can you make from the word equation?
- 8 What is word equation for class 10th?
- 9 What is the word equation for 2H2 O2 → 2H2O?
- 10 What is the word equation for calcium and water?
- 11 What is the word equation for potassium and water?
- 12 What is the word equation for sodium and chlorine?
- 13 How many words with or without meaning can be formed using the word EQUATION?
- 14 How many words without meaning can be formed using all the letters of the word EQUATION using each letter exactly once?
- 15 How many different words can be formed with the letters of the word EQUATION so that I the words begin with E?
- 16 Where do you write equations?
- 17 How do I write an equation in standard form?
What is a word equation give an example?
The chemical reaction. 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as. hydrogen gas + oxygen gas → steam. as a word equation or as “Hydrogen and oxygen react to form water” or “Water is made by reacting hydrogen and oxygen”.
What is a word equation and how it is written?
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation. ex: Cu + O2 → CuO.
What is simple equation?
A simple equation refers to a mathematical equation that expresses the relationship between two expressions on both sides of the ‘equal to’ sign. This category of an equation consists of a variable, usually in the form of x or y. Solving simple equations often require rearranging it.
What is word equation and how is it written class 10?
Hint: Word equation means we have to write the chemical reaction in the form of words. Chemical reaction means we have to mention the chemical symbols of the elements to represent the reaction. Nail contains iron in it. The molecular formula of copper sulphate is CuSO4.
What is the word equation for sodium and water?
sodium + water → sodium hydroxide + hydrogen.
How do you write an equation for a reaction?
A chemical equation consists of the chemical formulas of the reactants (on the left) and the products (on the right). The two are separated by an arrow symbol (“→” usually read aloud as “yields”). Each individual substance’s chemical formula is separated from others by a plus sign.
How many words can you make from the word equation?
109 words can be made from the letters in the word equation.
What is word equation for class 10th?
A word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants , products , and direction of the reaction in a form that could be used to write a chemical equation.
What is the word equation for 2H2 O2 → 2H2O?
The actual reaction to make water is a bit more complicated: 2H2 + O2 = 2H2O + Energy. In English, the equation says: To produce two molecules of water (H2O), two molecules of diatomic hydrogen (H2) must be combined with one molecule of diatomic oxygen (O2). Energy will be released in the process.
What is the word equation for calcium and water?
Ca+2H2O→Ca(OH)2+H2↑
What is the word equation for potassium and water?
How to Balance: K + H2O = KOH + H2| Breslyn.org.
What is the word equation for sodium and chlorine?
Na (s) + Cl2 (g) → NaCl (s)
Inspection of this equation, however, shows that, while there is one sodium atom on each side of the arrow, there are two chlorine atoms in the reactants and only one in the products.
How many words with or without meaning can be formed using the word EQUATION?
For x∈R,x=−1 if (1+x)2016+x(1+x)2015+x(1+x)2014+. +x2016=∑i=02016aixi, then a17 is equal to.
How many words without meaning can be formed using all the letters of the word EQUATION using each letter exactly once?
40,320
How many words, with or without meaning, can be formed using all the letters of the word EQUATION, using each letter exactly once? Summary: The number of words, with or without meaning, that can be formed using all the letters of the word EQUATION, using each letter exactly once is 40,320.
How many different words can be formed with the letters of the word EQUATION so that I the words begin with E?
6×720=4320.
Where do you write equations?
Write an equation or formula
- Choose Insert > Equation and choose the equation you want from the gallery.
- After you insert the equation the Equation Tools Design tab opens with symbols and structures that can be added to your equation.
How do I write an equation in standard form?
The standard form for linear equations in two variables is Ax+By=C. For example, 2x+3y=5 is a linear equation in standard form. When an equation is given in this form, it’s pretty easy to find both intercepts (x and y). This form is also very useful when solving systems of two linear equations.
Table of Contents
- How do you write and balance chemical equations?
- How do you do word equations?
- How do you write symbol equations?
- How do you change a formula into a word equation?
- What is a balanced equation in chemistry?
- What is the equation of rust?
- Do skeleton equations need to be balanced?
- What is the difference between a skeleton equation and a balanced equation?
- What is the skeletal equation for water?
- What is skeletal equation give example?
- What is the balanced equation of H2O?
- Which chemical equation is unbalanced?
- What type of reaction is H2O?
- Is 2H2 O2 2h20 a balanced equation?
- Is 4h2 2O2 4h2o balanced?
- What does the 4 in 4Na mean?
- Which of the following is not balanced equation?
- What is the balanced equation for heptane?
sodium + water → sodium hydroxide + hydrogen.
How do you write and balance chemical equations?
Summary
- To be useful, chemical equations must always be balanced. Balanced chemical equations have the same number and type of each atom on both sides of the equation.
- The coefficients in a balanced equation must be the simplest whole number ratio. Mass is always conserved in chemical reactions.
How do you do word equations?
Write an equation or formula
- Choose Insert > Equation and choose the equation you want from the gallery.
- After you insert the equation the Equation Tools Design tab opens with symbols and structures that can be added to your equation.
How do you write symbol equations?
Writing Chemical Equations
- In a chemical equation, the reactants are written on the left, and the products are written on the right.
- The coefficients next to the symbols of entities indicate the number of moles of a substance produced or used in the chemical reaction.
How do you change a formula into a word equation?
Step 1: Identify reactants and products and place them in a word equation. Step 2: Convert the chemical names into chemical formulas. Place them based on the chemical equation and write the state symbols. Step 3: Balance the chemical equation.
What is a balanced equation in chemistry?
A balanced chemical is equation has equal numbers of atoms for each element involved in the reaction are represented on the reactant and product sides. This is a requirement the equation must satisfy to be consistent with the law of conservation of matter.
What is the equation of rust?
Rust is apparently a hydrated form of iron(III)oxide. The formula is approximately Fe2O3•32H2O, although the exact amount of water is variable.
Do skeleton equations need to be balanced?
Chemistry students routinely use skeleton equations in order to balance the equations for chemical reactions. This is why it is called a “skeleton” equation. To make the equation complete, you need to solve for the correct coefficients for each of the chemicals, which indicate the relative amounts of each.
What is the difference between a skeleton equation and a balanced equation?
The key difference between balanced equation and skeleton equation is that balanced equation gives the actual number of molecules of each reactant and product involved in the chemical reaction whereas skeleton equation gives only the reactants of the reaction.
The following equation describes the burning of hydrogen gas to form liquid water. 2H2(g) + O2(g) → 2H2O(l ) Chemical equations give the following information about chemical reactions.
What is skeletal equation give example?
In skeletal chemical equation the mass no of elements in reactant side is not equal to the mass no of elements in product side. Later it has to be balanced by appropriate number of molecules. For example: Mg + O2 → MgO, it’s a skeleton equation. Balanced Equation : 2Mg(s) + O2(g) → 2MgO(s).
What is the balanced equation of H2O?
H2 + O2 → H2O. Word equation: Hydrogen gas + Oxygen gas → water. Type of Chemical Reaction: For this reaction we have a Combination reaction. Balancing Strategies: For this reaction it is helpful to start by changing the coefficient in front of H2O and so that you have an even number of oxygen atoms.
Which chemical equation is unbalanced?
According to the law of conservation of, the number of atoms in the products of a chemical reaction must be balanced with the number of atoms in the reactants. The chemical equation for the formation of ammonia is unbalanced.
What type of reaction is H2O?
A: Two hydrogen molecules per oxygen molecule are produced because water (H2O) has a ratio of two hydrogen atoms to one oxygen atom. A decomposition reaction occurs when one reactant breaks down into two or more products. This can be represented by the general equation: AB → A + B.
Is 2H2 O2 2h20 a balanced equation?
Yes, the equation is balanced. There are the same number of Hydrogen (H) and Oxygen (O) atoms on both sides of the equation.
Is 4h2 2O2 4h2o balanced?
Answer. yes hydrogen has 4×2=8 atoms and oxygen has 2×2=4 atoms on both sides hence it is balanced.
What does the 4 in 4Na mean?
As reactant you have 4Na = 4 atoms Na. As a product you have 2Na2 = 4 atoms Na. The substances sodium (Na), oxygen (O2), and sodium oxide (Na2O) participate in this chemical reaction: 4Na + O2 → 2Na2O. “Mass can be neither created nor destroyed”, therefore you can’t have more mass on one side than another.
Which of the following is not balanced equation?
The reaction of copper with nitric acid is not balanced because number of nitrogen, hydrogen and oxygen atom on both side is not equal.
What is the balanced equation for heptane?
1 Answer. Ernest Z. The balanced equation is C7H16+11O2→7CO2+8H2O .
Chemistry
Thomas Myers,Keith B. Oldham,Salvatore Tocci
2005 Edition
Chapter 8, Problem 16
Problem
For each of the following equations, write a sent…
03:40
Question
Answered step-by-step
Write the word equation for the electrolysis of water, and indicate the physical states and condition $(mathrm{s})$ of the reaction.
Answer
| Video Answer
Solved by verified expert
This problem has been solved!
Try Numerade free for 7 days
Ronald Prasad
Numerade Educator
Like
Report
| Textbook Answer
Official textbook answer
Video by Ronald Prasad
Numerade Educator
This textbook answer is only visible when subscribed! Please subscribe to view the answer
Recommended Videos
03:05
Write the word equation for t…
00:35
Write a balanced equation for the decomposition of water by electrolysis.
00:36
$$
text { Write equations for the electrolysis of } mathrm{CaH}_{2} text { in fused state. }
$$
01:44
Describe the electrolysis of aqueous sodium chloride. How do the products of the electrolysis compare for stirred and unstirred reactions? Write chem…
Transcript
Learning outcomes
At the end of this, you should be able to:
– State how you know a reaction has taken place
-Write a word equation for a given reaction
– Write a balanced symbol equation for a reaction, given the symbols for the reactants
– Write a balanced symbol equation for a reaction when you have to look up the symbols from a table and/or using the Periodic table (Higher only)
What you should already know
– What an element is– What a compound is– The difference between ionic and covalent compounds– What a molecule is– How to write the formula for a molecule or compound using symbols
Word equations and balanced symbol equations
A reaction may happen when two or more chemicals are mixed. Sometimes you have to add heat (energy) before the reaction will occur.
Not all chemicals react together when they are mixed. We can tell a reaction has taken place if we see bubbles of gas, a change in colour, a change in temperature or a precipitate (a layer of solid in the bottom of the test tube that wasn’t there when you started). So vinegar (an acid) reacts with sodium bicarbonate – there are bubbles of gas and the test tube gets warm – but it doesn’t react with table salt (sodium chloride). The salt dissolves but nothing else happens, even if you heat the mixture.
A word equation helps us work out what has happened during a reaction.
Let’s look at the word equation for the reaction between sulphuric acid and calcium carbonate (limestone or marble).
We know a reaction happens because we see bubbles of gas and the test tube gets warm.
The clue to what the gas is is in the name of the reactants; if you add acid to a carbonate you always get carbon dioxide. (You could also test the gas; we know it is carbon dioxide because if we bubble it through limewater, the limewater goes ‘milky’.) In addition, if you react an acid with an alkali or base, like a carbonate, an oxide or a hydroxide, you always get water. (You need to know this for the chemistry exam.) The name of the acid gives you a clue to the name of the salt it forms.
Calcium carbonate + sulphuric acid→ calcium sulfate + carbon dioxide + water
Let’s look at another reaction. This is one you learn about in biology; respiration.
The reactants are glucose and oxygen, the products are carbon dioxide and water.
Glucose + oxygen→ carbon dioxide + water (+energy)
(This is almost the same as burning a hydrocarbon; you get the same products.)
Some reactions are very hard to describe in any other way than by a word equation. Not all substances have a chemical formula you can write down, for example, wood or a chocolate biscuit. Or the chemical formula may be one you don’t know, such as vinegar which is mainly a solution of something called ethanoic acid.
Word equations describe what is happening, but it is often more useful to know what each of the atoms, molecules or ions is doing. To do this, we use a balanced symbol equation. Sometimes you will have to look up the formulas for the compounds or even work them out using the Periodic Table.
‘Balancing’ just means making sure you have the same number of each sort of atom on each side.
This is important. We do not lose or gain mass during a chemical reaction. This is called ‘Conservation of mass’. You may have come across this idea already in Physics.
Let’s see how to write a balanced formula equation for the reaction between magnesium and hydrochloric acid. You know a reaction happens because there is fizzing. If you test the gas with a lighted splint, you get a ‘squeaky pop’, telling you hydrogen has been produced.
The word equation is
magnesium + hydrochloric acid → hydrogen + magnesium chloride.
If you look in the table of ions, you will see that magnesium makes ++ ions and chloride ions are – , so you need 2 chlorine ions to each ion of magnesium. You could also work this out by looking at the Periodic Table. Magnesium is in the second column of the Periodic table and chlorine is in the next-to-last column, so magnesium has 2 electrons in its outer shell to give away, and chlorine has 7 electrons in its outer shell, so needs 1 to make a full shell.
To write the symbol equation, first write down the reactants and products in symbol form: (mathrm{Mg}+mathrm{HCl} rightarrow mathrm{MgCI}_{2}+mathrm{H}_{2})
This doesn’t balance; we have 1 H and 1 Cl on the left-hand side and two of each on the right-hand side. So we need to add another HCl to make the equation balance:
(mathrm{Mg}+2 mathrm{HCl} rightarrow mathrm{MgCI}_{2}+mathrm{H}_{2})
The hydrochloric acid is in solution, and so is the magnesium chloride. You can show this by writing a little ‘aq’ (short for aqueous, which means ‘in water’) under the HCl and (mathrm{MgCI}_{2}). You could also show that the magnesium chloride is ionic and in solution by writing (mathrm{Mg}++(mathrm{Cl}-)_{2}) instead of MgCl.
You have probably seen the reaction between a group I metal and water; for example sodium and water. The sodium races around fizzing and there is a yellow flame too.
The fizzing tells us there is a gas being produced. The flame tells us there is also a lot of heat being produced; enough to ignite the gas. There is definitely a reaction going on!
The only substances involved are sodium and water and if you test the water afterwards with pH or litmus paper, you will find it is alkaline. The gas gives a ‘squeaky pop’ if it is tested with a lighted splint; it must be hydrogen.
Sodium + water→ hydrogen + sodium hydroxide.
In symbols:
(mathrm{Na}+mathrm{H}_{2} mathrm{O} rightarrow mathrm{H}_{2}+mathrm{NaOH})
This doesn’t balance, there are too many H’s on the right-hand side. If we add another Na, we also need another (mathrm{H}_{2} mathrm{O}) to make sure we have enough OH.
So we have:
(2 mathrm{Na}+2 mathrm{H}_{2} mathrm{O} rightarrow 2 mathrm{Na} mathrm{OH}+mathrm{H}_{2})
This is an example of a very important reaction; make sure you learn it, and the one for Group II metals. You’ll find that in the ‘some to try for yourself’ section.
Another reaction you might have seen or done is the reaction between copper II oxide and dilute sulphuric acid. This is a reaction requiring heat (and a lot of stirring) to make it happen. Copper II oxide (CuO) is a black insoluble powder. Dilute sulphuric is a clear liquid. The products are copper II sulfate and water. Copper II sulfate is a pretty blue colour. You can tell this reaction has happened because you have a change in colour.
Copper II oxide + dilute sulphuric acid → copper sulfate + water
Write the symbols:
(mathrm{Cu} mathrm{O}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{CuSO}_{4}+mathrm{H}_{2} mathrm{O})
There is the same number of atoms on each side; the equation is balanced. The dilute sulphuric acid and copper sulfate are both in solution, so you could write ‘aq’ underneath or show them as ions.
Let’s look at something which needs more balancing: the reaction between iron and chlorine. These are both elements and you aren’t adding anything else, so the product will be iron III chlorides, as long as you have lots of chlorine. Chlorine makes Cl- ions and iron III ions are +++.
Chlorine always comes as Cl2 molecules.
Iron + chlorine → iron III chloride
Write a formula equation:
(mathrm{Fe}+mathrm{CI}_{2} rightarrow mathrm{FeCI}_{3})
This doesn’t balance: you have 2 chlorines on the left-hand side and 3 on the right-hand side. You need to use a bit of clever maths here; 2 x 3 = 6. If you put a 3 in front of the (mathrm{CI}_{2}) and a 2 in front of the (mathrm{FeCI}_{3}), you have 6 chlorines on both sides. However, you now also have to put a 2 in front of the Fe on the left-hand side because you have 2 irons on the right-hand side:
(2 mathrm{Fe}+3 mathrm{CI}_{2} rightarrow 2 mathrm{FeCI}_{3})
You will find this ‘balancing trick’ with 2 and 3 useful for a lot of iron and also aluminium compounds. (The only — ion you are likely to come across is phosphate.) There is one to try in the ‘some to try for yourself’ section. But first here’s another example.
Let’s look at the reaction between aluminium hydroxide and sulphuric acid.It’s a reaction between an alkali and an acid, so we will get water as well as salt.
The salt is aluminium sulfate, and we can use the balancing trick to work out it is (mathrm{AI}_{2}left(mathrm{SO}_{4}right)_{3}).
Aluminium hydroxide + sulphuric acid → aluminium sulfate + water.
Begin by writing down the symbols:
(mathrm{Al}(mathrm{OH})_{3}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right)_{3}+mathrm{H}_{2} mathrm{O})
We can see straight away that we need 2 aluminium ions and 3 molecules of sulphuric acid on the left-hand side to make the aluminium sulfate.
(2 mathrm{Al}(mathrm{OH})_{3}+3 mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right) 3+mathrm{H}_{2} mathrm{O})
Now we have 6 OH’s and 6 H’s on the left. Each O needs 2 H’s to make water, so we have the right amount to make 6 (mathrm{H}_{2} mathrm{O}) with nothing left over.
The final balanced equation is:
(2 mathrm{Al}(mathrm{OH})_{3}+3 mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right)_{3}+6 mathrm{H}_{2} mathrm{O})
Take your time and remember to make sure you have the same number of each element or ion on each side.
You might find it helps to write the symbols in groups and cross them off as you use them:
These symbol equations all involve elements and ions, but we can write balanced symbol equations for other reactions, too.
If we burn the hydrocarbon gas propane in oxygen (or in the air), we get carbon dioxide and water.
Propane + oxygen → carbon dioxide + water
The formula for propane is (mathrm{C}_{3} mathrm{H}_{8}).
Write down a symbol equation:
(mathrm{C}_{3} mathrm{H}_{8}+mathrm{O}_{2} rightarrow mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
You can see straight away that there are 3C’s on the left and 1 on the right, so you know there must be 3 (mathrm{CO}_{2}) ‘s. The 8 H’s will make 4 water molecules. So you need 4O’s for the water and 6 for the (mathrm{CO}_{2}) ‘s; 10 O’s or (5 mathrm{O}_{2}) ‘s.
The balanced equation is:
(mathrm{C}_{3} mathrm{H}_{8}+5 mathrm{O}_{2} rightarrow 3 mathrm{CO}_{2}+4 mathrm{H}_{2} mathrm{O})
Sometimes you end up with an odd number of O’s or H’s. If this happens, you just double everything up.
Propane’s cousin ethane C2H6 needs this:
(2 mathrm{C}_{2} mathrm{H}_{6}+7 mathrm{O}_{2} rightarrow 4 mathrm{CO}_{2}+6 mathrm{H}_{2} mathrm{O})
Summary
- You know a reaction has happened because you get bubbles of gas, a change in colour, a change in temperature or a precipitate.
- You can write a reaction as a word equation. This is useful if you don’t know the formula or for complicated compounds/substances.
- You can also write a reaction as a balanced symbol equation. Balanced means having the same number of atoms of each element on each side of the equation.
- You might need to use the Periodic Table or a table of ions to work out the formulas of the things you are reacting.
- You need to know what happens when you react an acid with an oxide, a hydroxide or a carbonate.
- You need to know what happens when you react to a Group I or II metal with water.
- You need to know what happens when you burn a hydrocarbon.
- You need to be able to write word equations for respiration and photosynthesis.
Some to try for yourself
- Write a word equation for the reaction that occurs when a candle is burned. (Candle wax is a hydrocarbon.)
- Write a word equation for the reaction between potassium and water.
- Write a balanced formula equation for the reaction between calcium metal and water.
- Write a balanced formula equation for the reaction between magnesium carbonate (left(mathrm{MgCO}_{3}right)) and sulphuric acid (left(mathrm{H}_{2} mathrm{SO}_{4}right)).
- Write a balanced formula equation for the reaction between iron II oxide (FeO) and nitric acid (left(mathrm{HNO}_{3}right)).
- Write a balanced formula equation for the reaction between sodium carbonate and hydrochloric acid.
- Ammonia (left(mathrm{NH}_{3}right)) is made by reacting together nitrogen and hydrogen.
- Write a balanced formula equation for this reaction.
- Iron is extracted from haematite iron ore (Iron III oxide) by heating with carbon. Write a balanced formula equation for this reaction. Write a balanced formula equation for the reaction between aluminium oxide and nitric acid.
- Quicklime is made by heating limestone, calcium carbonate. A gas is given off during the reaction. Quicklime is alkaline and reacts with water to form calcium hydroxide. Write balanced formula equations for these two reactions?
Answers
Remember to write a word equation before you write the balanced formula equation to make sure you don’t forget anything.
Answer 1
candle wax + oxygen → carbon dioxide + water
Answer 2
potassium + water → potassium hydroxide + hydrogen
Answer 3
calcium + water → calcium hydroxide + hydrogen
(mathrm{Ca}+2 mathrm{H}_{2} mathrm{O} rightarrow mathrm{Ca}(mathrm{OH})_{2}+mathrm{H}_{2})
Calcium is Group II so it is ++. Otherwise, it’s the same as the reaction between sodium and water. The calcium fizzes and the test tube gets warm.
Answer 4
magnesium carbonate + sulphuric acid → magnesium sulfate + carbon dioxide + water
(mathrm{MgCO}_{3}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{MgSO}_{4}+mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
In the acid/carbonate reaction, the carbonate ion gives an oxygen atom to the hydrogen from the acid to form a water molecule.
Magnesium carbonate is the chemical name for gypsum, a soft white rock.
Answer 5
Iron II oxide + nitric acid → iron II nitrate + water
(mathrm{FeO}+2 mathrm{HNO}_{3} rightarrow mathrm{Fe}left(mathrm{NO}_{3}right)_{2}+mathrm{H}_{2} mathrm{O})
Transition metals like iron and copper often have more than one ‘oxidation state’ – they can have different numbers of +. Watch out for things like iron II and copper I.
Answer 6
Sodium carbonate + hydrochloric acid → sodium chloride + carbon dioxide + water
(mathrm{Na}_{2} mathrm{CO}_{3}+2 mathrm{HCl} rightarrow 2 mathrm{NaCl}+mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
Something alkaline + an acid gives a salt. This reaction gives ‘table salt’. Sodium carbonate is washing soda.
Answer 7
(mathrm{N}_{2}+3 mathrm{H}_{2} rightarrow 2 mathrm{NH}_{3})
This is an important reaction because it is used to make ammonia for fertiliser.It is what is called an ‘equilibrium’ reaction; not all the reactants are turned into a product. You will learn more about this reaction in a later unit of your course.
Answer 8
Iron III oxide + carbon → iron + carbon dioxide
(2 mathrm{Fe}_{2} mathrm{O}_{3}+3 mathrm{C} rightarrow 2 mathrm{Fe}+3 mathrm{CO}_{2})
You will learn about the extraction of metals later in your course.
Answer 9
Aluminium oxide + nitric acid → aluminium nitrate + water
(mathrm{Al}_{2} mathrm{O}_{3}+6 mathrm{HNO}_{3} rightarrow 2 mathrm{Al}left(mathrm{NO}_{3}right)_{3}+3 mathrm{H}_{2} mathrm{O})
Aluminium reacts in a similar way to group II metals but forms +++ ions.
Since there are 2 Al ions there need to be (6 mathrm{NO}_{3}) ions.
Answer 10
The gas given off is carbon dioxide. This means that the first reaction is
(mathrm{CaCO}_{3} rightarrow mathrm{CO}_{2}+mathrm{CaO})
This tells you that quicklime is calcium oxide.
The calcium oxide is reacted with water. Nothing is given off, so the product must be calcium hydroxide. This is known as slaked lime.
(mathrm{CaO}+mathrm{H}_{2} mathrm{O} rightarrow mathrm{Ca}(mathrm{OH})_{2})
These are very important reactions.
Calcium oxide is used in the manufacture of cement, concrete and mortar (used to stick bricks together). A lot of heat is given out by the second reaction (it is exothermic) – if you’ve ever mixed cement you will know it gets hot. This is why.
Lime is also used to ‘sweeten’ clay soils to make them easier to work and more productive.
Slaked lime is dissolved in water to make lime water, which you use to test for carbon dioxide. The milkiness is due to the particles of insoluble calcium carbonate formed when calcium hydroxide reacts with carbon dioxide.
(mathrm{Ca}(mathrm{OH})_{2}+mathrm{CO}_{2} rightarrow mathrm{CaCO}_{3}+mathrm{H}_{2} mathrm{O})