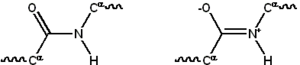Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.
A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; but in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by post-translational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Some proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.
Once formed, proteins only exist for a certain period and are then degraded and recycled by the cell’s machinery through the process of protein turnover. A protein’s lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.
Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyse biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. In animals, proteins are needed in the diet to provide the essential amino acids that cannot be synthesized. Digestion breaks the proteins down for metabolic use.
Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.
History and etymology
Proteins were recognized as a distinct class of biological molecules in the eighteenth century by Antoine Fourcroy and others, distinguished by the molecules’ ability to coagulate or flocculate under treatments with heat or acid.[1] Noted examples at the time included albumin from egg whites, blood serum albumin, fibrin, and wheat gluten.
Proteins were first described by the Dutch chemist Gerardus Johannes Mulder and named by the Swedish chemist Jöns Jacob Berzelius in 1838.[2][3] Mulder carried out elemental analysis of common proteins and found that nearly all proteins had the same empirical formula, C400H620N100O120P1S1.[4] He came to the erroneous conclusion that they might be composed of a single type of (very large) molecule. The term «protein» to describe these molecules was proposed by Mulder’s associate Berzelius; protein is derived from the Greek word πρώτειος (proteios), meaning «primary»,[5] «in the lead», or «standing in front»,[6] + -in. Mulder went on to identify the products of protein degradation such as the amino acid leucine for which he found a (nearly correct) molecular weight of 131 Da.[4] Prior to «protein», other names were used, like «albumins» or «albuminous materials» (Eiweisskörper, in German).[7]
Early nutritional scientists such as the German Carl von Voit believed that protein was the most important nutrient for maintaining the structure of the body, because it was generally believed that «flesh makes flesh.»[8] Karl Heinrich Ritthausen extended known protein forms with the identification of glutamic acid. At the Connecticut Agricultural Experiment Station a detailed review of the vegetable proteins was compiled by Thomas Burr Osborne. Working with Lafayette Mendel and applying Liebig’s law of the minimum in feeding laboratory rats, the nutritionally essential amino acids were established. The work was continued and communicated by William Cumming Rose. The understanding of proteins as polypeptides came through the work of Franz Hofmeister and Hermann Emil Fischer in 1902.[9][10] The central role of proteins as enzymes in living organisms was not fully appreciated until 1926, when James B. Sumner showed that the enzyme urease was in fact a protein.[11]
The difficulty in purifying proteins in large quantities made them very difficult for early protein biochemists to study. Hence, early studies focused on proteins that could be purified in large quantities, e.g., those of blood, egg white, various toxins, and digestive/metabolic enzymes obtained from slaughterhouses. In the 1950s, the Armour Hot Dog Co. purified 1 kg of pure bovine pancreatic ribonuclease A and made it freely available to scientists; this gesture helped ribonuclease A become a major target for biochemical study for the following decades.[4]
Linus Pauling is credited with the successful prediction of regular protein secondary structures based on hydrogen bonding, an idea first put forth by William Astbury in 1933.[12] Later work by Walter Kauzmann on denaturation,[13][14] based partly on previous studies by Kaj Linderstrøm-Lang,[15] contributed an understanding of protein folding and structure mediated by hydrophobic interactions.
The first protein to be sequenced was insulin, by Frederick Sanger, in 1949. Sanger correctly determined the amino acid sequence of insulin, thus conclusively demonstrating that proteins consisted of linear polymers of amino acids rather than branched chains, colloids, or cyclols.[16] He won the Nobel Prize for this achievement in 1958.[17]
With the development of X-ray crystallography, it became possible to sequence protein structures.[18] The first protein structures to be solved were hemoglobin by Max Perutz and myoglobin by John Kendrew, in 1958.[19][20] The use of computers and increasing computing power also supported the sequencing of complex proteins. In 1999, Roger Kornberg succeeded in sequencing the highly complex structure of RNA polymerase using high intensity X-rays from synchrotrons.[18]
Since then, cryo-electron microscopy (cryo-EM) of large macromolecular assemblies[21] has been developed. Cryo-EM uses protein samples that are frozen rather than crystals, and beams of electrons rather than x-rays. It causes less damage to the sample, allowing scientists to obtain more information and analyze larger structures.[18] Computational protein structure prediction of small protein domains[22] has also helped researchers to approach atomic-level resolution of protein structures.
As of 2017, the Protein Data Bank has over 126,060 atomic-resolution structures of proteins.[23]
Number of proteins encoded in genomes
The number of proteins encoded in a genome roughly corresponds to the number of genes (although there may be a significant number of genes that encode RNA of protein, e.g. ribosomal RNAs). Viruses typically encode a few to a few hundred proteins, archaea and bacteria a few hundred to a few thousand, while eukaryotes typically encode a few thousand up to tens of thousands of proteins (see genome size for a list of examples).
Biochemistry
Chemical structure of the peptide bond (bottom) and the three-dimensional structure of a peptide bond between an alanine and an adjacent amino acid (top/inset). The bond itself is made of the CHON elements.
Most proteins consist of linear polymers built from series of up to 20 different L-α- amino acids. All proteinogenic amino acids possess common structural features, including an α-carbon to which an amino group, a carboxyl group, and a variable side chain are bonded. Only proline differs from this basic structure as it contains an unusual ring to the N-end amine group, which forces the CO–NH amide moiety into a fixed conformation.[24] The side chains of the standard amino acids, detailed in the list of standard amino acids, have a great variety of chemical structures and properties; it is the combined effect of all of the amino acid side chains in a protein that ultimately determines its three-dimensional structure and its chemical reactivity.[25]
The amino acids in a polypeptide chain are linked by peptide bonds. Once linked in the protein chain, an individual amino acid is called a residue, and the linked series of carbon, nitrogen, and oxygen atoms are known as the main chain or protein backbone.[26]: 19
The peptide bond has two resonance forms that contribute some double-bond character and inhibit rotation around its axis, so that the alpha carbons are roughly coplanar. The other two dihedral angles in the peptide bond determine the local shape assumed by the protein backbone.[26]: 31 The end with a free amino group is known as the N-terminus or amino terminus, whereas the end of the protein with a free carboxyl group is known as the C-terminus or carboxy terminus (the sequence of the protein is written from N-terminus to C-terminus, from left to right).
The words protein, polypeptide, and peptide are a little ambiguous and can overlap in meaning. Protein is generally used to refer to the complete biological molecule in a stable conformation, whereas peptide is generally reserved for a short amino acid oligomers often lacking a stable 3D structure. But the boundary between the two is not well defined and usually lies near 20–30 residues.[27] Polypeptide can refer to any single linear chain of amino acids, usually regardless of length, but often implies an absence of a defined conformation.
Interactions
Proteins can interact with many types of molecules, including with other proteins, with lipids, with carbohydrates, and with DNA.[28][29][26][30]
Abundance in cells
It has been estimated that average-sized bacteria contain about 2 million proteins per cell (e.g. E. coli and Staphylococcus aureus). Smaller bacteria, such as Mycoplasma or spirochetes contain fewer molecules, on the order of 50,000 to 1 million. By contrast, eukaryotic cells are larger and thus contain much more protein. For instance, yeast cells have been estimated to contain about 50 million proteins and human cells on the order of 1 to 3 billion.[31] The concentration of individual protein copies ranges from a few molecules per cell up to 20 million.[32] Not all genes coding proteins are expressed in most cells and their number depends on, for example, cell type and external stimuli. For instance, of the 20,000 or so proteins encoded by the human genome, only 6,000 are detected in lymphoblastoid cells.[33]
Synthesis
Biosynthesis
A ribosome produces a protein using mRNA as template
The DNA sequence of a gene encodes the amino acid sequence of a protein
Proteins are assembled from amino acids using information encoded in genes. Each protein has its own unique amino acid sequence that is specified by the nucleotide sequence of the gene encoding this protein. The genetic code is a set of three-nucleotide sets called codons and each three-nucleotide combination designates an amino acid, for example AUG (adenine–uracil–guanine) is the code for methionine. Because DNA contains four nucleotides, the total number of possible codons is 64; hence, there is some redundancy in the genetic code, with some amino acids specified by more than one codon.[30]: 1002–42 Genes encoded in DNA are first transcribed into pre-messenger RNA (mRNA) by proteins such as RNA polymerase. Most organisms then process the pre-mRNA (also known as a primary transcript) using various forms of Post-transcriptional modification to form the mature mRNA, which is then used as a template for protein synthesis by the ribosome. In prokaryotes the mRNA may either be used as soon as it is produced, or be bound by a ribosome after having moved away from the nucleoid. In contrast, eukaryotes make mRNA in the cell nucleus and then translocate it across the nuclear membrane into the cytoplasm, where protein synthesis then takes place. The rate of protein synthesis is higher in prokaryotes than eukaryotes and can reach up to 20 amino acids per second.[34]
The process of synthesizing a protein from an mRNA template is known as translation. The mRNA is loaded onto the ribosome and is read three nucleotides at a time by matching each codon to its base pairing anticodon located on a transfer RNA molecule, which carries the amino acid corresponding to the codon it recognizes. The enzyme aminoacyl tRNA synthetase «charges» the tRNA molecules with the correct amino acids. The growing polypeptide is often termed the nascent chain. Proteins are always biosynthesized from N-terminus to C-terminus.[30]: 1002–42
The size of a synthesized protein can be measured by the number of amino acids it contains and by its total molecular mass, which is normally reported in units of daltons (synonymous with atomic mass units), or the derivative unit kilodalton (kDa). The average size of a protein increases from Archaea to Bacteria to Eukaryote (283, 311, 438 residues and 31, 34, 49 kDa respectively) due to a bigger number of protein domains constituting proteins in higher organisms.[35] For instance, yeast proteins are on average 466 amino acids long and 53 kDa in mass.[27] The largest known proteins are the titins, a component of the muscle sarcomere, with a molecular mass of almost 3,000 kDa and a total length of almost 27,000 amino acids.[36]
Chemical synthesis
Short proteins can also be synthesized chemically by a family of methods known as peptide synthesis, which rely on organic synthesis techniques such as chemical ligation to produce peptides in high yield.[37] Chemical synthesis allows for the introduction of non-natural amino acids into polypeptide chains, such as attachment of fluorescent probes to amino acid side chains.[38] These methods are useful in laboratory biochemistry and cell biology, though generally not for commercial applications. Chemical synthesis is inefficient for polypeptides longer than about 300 amino acids, and the synthesized proteins may not readily assume their native tertiary structure. Most chemical synthesis methods proceed from C-terminus to N-terminus, opposite the biological reaction.[39]
Structure
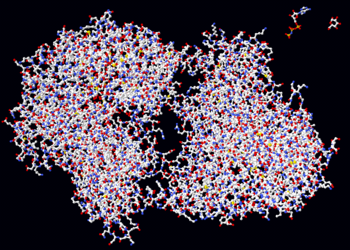
The crystal structure of the chaperonin, a huge protein complex. A single protein subunit is highlighted. Chaperonins assist protein folding.
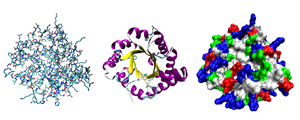
Three possible representations of the three-dimensional structure of the protein triose phosphate isomerase. Left: All-atom representation colored by atom type. Middle: Simplified representation illustrating the backbone conformation, colored by secondary structure. Right: Solvent-accessible surface representation colored by residue type (acidic residues red, basic residues blue, polar residues green, nonpolar residues white).
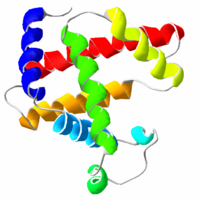
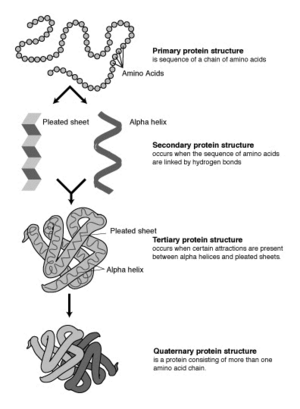
Most proteins fold into unique 3D structures. The shape into which a protein naturally folds is known as its native conformation.[26]: 36 Although many proteins can fold unassisted, simply through the chemical properties of their amino acids, others require the aid of molecular chaperones to fold into their native states.[26]: 37 Biochemists often refer to four distinct aspects of a protein’s structure:[26]: 30–34
- Primary structure: the amino acid sequence. A protein is a polyamide.
- Secondary structure: regularly repeating local structures stabilized by hydrogen bonds. The most common examples are the α-helix, β-sheet and turns. Because secondary structures are local, many regions of different secondary structure can be present in the same protein molecule.
- Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structures to one another. Tertiary structure is generally stabilized by nonlocal interactions, most commonly the formation of a hydrophobic core, but also through salt bridges, hydrogen bonds, disulfide bonds, and even posttranslational modifications. The term «tertiary structure» is often used as synonymous with the term fold. The tertiary structure is what controls the basic function of the protein.
- Quaternary structure: the structure formed by several protein molecules (polypeptide chains), usually called protein subunits in this context, which function as a single protein complex.
- Quinary structure: the signatures of protein surface that organize the crowded cellular interior. Quinary structure is dependent on transient, yet essential, macromolecular interactions that occur inside living cells.
Proteins are not entirely rigid molecules. In addition to these levels of structure, proteins may shift between several related structures while they perform their functions. In the context of these functional rearrangements, these tertiary or quaternary structures are usually referred to as «conformations», and transitions between them are called conformational changes. Such changes are often induced by the binding of a substrate molecule to an enzyme’s active site, or the physical region of the protein that participates in chemical catalysis. In solution proteins also undergo variation in structure through thermal vibration and the collision with other molecules.[30]: 368–75
Proteins can be informally divided into three main classes, which correlate with typical tertiary structures: globular proteins, fibrous proteins, and membrane proteins. Almost all globular proteins are soluble and many are enzymes. Fibrous proteins are often structural, such as collagen, the major component of connective tissue, or keratin, the protein component of hair and nails. Membrane proteins often serve as receptors or provide channels for polar or charged molecules to pass through the cell membrane.[30]: 165–85
A special case of intramolecular hydrogen bonds within proteins, poorly shielded from water attack and hence promoting their own dehydration, are called dehydrons.[40]
Protein domains
Many proteins are composed of several protein domains, i.e. segments of a protein that fold into distinct structural units. Domains usually also have specific functions, such as enzymatic activities (e.g. kinase) or they serve as binding modules (e.g. the SH3 domain binds to proline-rich sequences in other proteins).
Sequence motif
Short amino acid sequences within proteins often act as recognition sites for other proteins.[41] For instance, SH3 domains typically bind to short PxxP motifs (i.e. 2 prolines [P], separated by two unspecified amino acids [x], although the surrounding amino acids may determine the exact binding specificity). Many such motifs has been collected in the Eukaryotic Linear Motif (ELM) database.
Protein topology
Topology of a protein describes the entanglement of the backbone and the arrangement of contacts within the folded chain.[42] Two theoretical frameworks of knot theory and Circuit topology have been applied to characterise protein topology. Being able to describe protein topology opens up new pathways for protein engineering and pharmaceutical development, and adds to our understanding of protein misfolding diseases such as neuromuscular disorders and cancer.
Cellular functions
Proteins are the chief actors within the cell, said to be carrying out the duties specified by the information encoded in genes.[27] With the exception of certain types of RNA, most other biological molecules are relatively inert elements upon which proteins act. Proteins make up half the dry weight of an Escherichia coli cell, whereas other macromolecules such as DNA and RNA make up only 3% and 20%, respectively.[43] The set of proteins expressed in a particular cell or cell type is known as its proteome.
The enzyme hexokinase is shown as a conventional ball-and-stick molecular model. To scale in the top right-hand corner are two of its substrates, ATP and glucose.
The chief characteristic of proteins that also allows their diverse set of functions is their ability to bind other molecules specifically and tightly. The region of the protein responsible for binding another molecule is known as the binding site and is often a depression or «pocket» on the molecular surface. This binding ability is mediated by the tertiary structure of the protein, which defines the binding site pocket, and by the chemical properties of the surrounding amino acids’ side chains. Protein binding can be extraordinarily tight and specific; for example, the ribonuclease inhibitor protein binds to human angiogenin with a sub-femtomolar dissociation constant (<10−15 M) but does not bind at all to its amphibian homolog onconase (>1 M). Extremely minor chemical changes such as the addition of a single methyl group to a binding partner can sometimes suffice to nearly eliminate binding; for example, the aminoacyl tRNA synthetase specific to the amino acid valine discriminates against the very similar side chain of the amino acid isoleucine.[44]
Proteins can bind to other proteins as well as to small-molecule substrates. When proteins bind specifically to other copies of the same molecule, they can oligomerize to form fibrils; this process occurs often in structural proteins that consist of globular monomers that self-associate to form rigid fibers. Protein–protein interactions also regulate enzymatic activity, control progression through the cell cycle, and allow the assembly of large protein complexes that carry out many closely related reactions with a common biological function. Proteins can also bind to, or even be integrated into, cell membranes. The ability of binding partners to induce conformational changes in proteins allows the construction of enormously complex signaling networks.[30]: 830–49
As interactions between proteins are reversible, and depend heavily on the availability of different groups of partner proteins to form aggregates that are capable to carry out discrete sets of function, study of the interactions between specific proteins is a key to understand important aspects of cellular function, and ultimately the properties that distinguish particular cell types.[45][46]
Enzymes
The best-known role of proteins in the cell is as enzymes, which catalyse chemical reactions. Enzymes are usually highly specific and accelerate only one or a few chemical reactions. Enzymes carry out most of the reactions involved in metabolism, as well as manipulating DNA in processes such as DNA replication, DNA repair, and transcription. Some enzymes act on other proteins to add or remove chemical groups in a process known as posttranslational modification. About 4,000 reactions are known to be catalysed by enzymes.[47] The rate acceleration conferred by enzymatic catalysis is often enormous—as much as 1017-fold increase in rate over the uncatalysed reaction in the case of orotate decarboxylase (78 million years without the enzyme, 18 milliseconds with the enzyme).[48]
The molecules bound and acted upon by enzymes are called substrates. Although enzymes can consist of hundreds of amino acids, it is usually only a small fraction of the residues that come in contact with the substrate, and an even smaller fraction—three to four residues on average—that are directly involved in catalysis.[49] The region of the enzyme that binds the substrate and contains the catalytic residues is known as the active site.
Dirigent proteins are members of a class of proteins that dictate the stereochemistry of a compound synthesized by other enzymes.[50]
Cell signaling and ligand binding
Many proteins are involved in the process of cell signaling and signal transduction. Some proteins, such as insulin, are extracellular proteins that transmit a signal from the cell in which they were synthesized to other cells in distant tissues. Others are membrane proteins that act as receptors whose main function is to bind a signaling molecule and induce a biochemical response in the cell. Many receptors have a binding site exposed on the cell surface and an effector domain within the cell, which may have enzymatic activity or may undergo a conformational change detected by other proteins within the cell.[29]: 251–81
Antibodies are protein components of an adaptive immune system whose main function is to bind antigens, or foreign substances in the body, and target them for destruction. Antibodies can be secreted into the extracellular environment or anchored in the membranes of specialized B cells known as plasma cells. Whereas enzymes are limited in their binding affinity for their substrates by the necessity of conducting their reaction, antibodies have no such constraints. An antibody’s binding affinity to its target is extraordinarily high.[30]: 275–50
Many ligand transport proteins bind particular small biomolecules and transport them to other locations in the body of a multicellular organism. These proteins must have a high binding affinity when their ligand is present in high concentrations, but must also release the ligand when it is present at low concentrations in the target tissues. The canonical example of a ligand-binding protein is haemoglobin, which transports oxygen from the lungs to other organs and tissues in all vertebrates and has close homologs in every biological kingdom.[30]: 222–29 Lectins are sugar-binding proteins which are highly specific for their sugar moieties. Lectins typically play a role in biological recognition phenomena involving cells and proteins.[51] Receptors and hormones are highly specific binding proteins.
Transmembrane proteins can also serve as ligand transport proteins that alter the permeability of the cell membrane to small molecules and ions. The membrane alone has a hydrophobic core through which polar or charged molecules cannot diffuse. Membrane proteins contain internal channels that allow such molecules to enter and exit the cell. Many ion channel proteins are specialized to select for only a particular ion; for example, potassium and sodium channels often discriminate for only one of the two ions.[29]: 232–34
Structural proteins
Structural proteins confer stiffness and rigidity to otherwise-fluid biological components. Most structural proteins are fibrous proteins; for example, collagen and elastin are critical components of connective tissue such as cartilage, and keratin is found in hard or filamentous structures such as hair, nails, feathers, hooves, and some animal shells.[30]: 178–81 Some globular proteins can also play structural functions, for example, actin and tubulin are globular and soluble as monomers, but polymerize to form long, stiff fibers that make up the cytoskeleton, which allows the cell to maintain its shape and size.
Other proteins that serve structural functions are motor proteins such as myosin, kinesin, and dynein, which are capable of generating mechanical forces. These proteins are crucial for cellular motility of single celled organisms and the sperm of many multicellular organisms which reproduce sexually. They also generate the forces exerted by contracting muscles[30]: 258–64, 272 and play essential roles in intracellular transport.
Protein evolution
A key question in molecular biology is how proteins evolve, i.e. how can mutations (or rather changes in amino acid sequence) lead to new structures and functions? Most amino acids in a protein can be changed without disrupting activity or function, as can be seen from numerous homologous proteins across species (as collected in specialized databases for protein families, e.g. PFAM).[52] In order to prevent dramatic consequences of mutations, a gene may be duplicated before it can mutate freely. However, this can also lead to complete loss of gene function and thus pseudo-genes.[53] More commonly, single amino acid changes have limited consequences although some can change protein function substantially, especially in enzymes. For instance, many enzymes can change their substrate specificity by one or a few mutations.[54] Changes in substrate specificity are facilitated by substrate promiscuity, i.e. the ability of many enzymes to bind and process multiple substrates. When mutations occur, the specificity of an enzyme can increase (or decrease) and thus its enzymatic activity.[54] Thus, bacteria (or other organisms) can adapt to different food sources, including unnatural substrates such as plastic.[55]
Methods of study
The activities and structures of proteins may be examined in vitro, in vivo, and in silico. In vitro studies of purified proteins in controlled environments are useful for learning how a protein carries out its function: for example, enzyme kinetics studies explore the chemical mechanism of an enzyme’s catalytic activity and its relative affinity for various possible substrate molecules. By contrast, in vivo experiments can provide information about the physiological role of a protein in the context of a cell or even a whole organism. In silico studies use computational methods to study proteins.
Protein purification
To perform in vitro analysis, a protein must be purified away from other cellular components. This process usually begins with cell lysis, in which a cell’s membrane is disrupted and its internal contents released into a solution known as a crude lysate. The resulting mixture can be purified using ultracentrifugation, which fractionates the various cellular components into fractions containing soluble proteins; membrane lipids and proteins; cellular organelles, and nucleic acids. Precipitation by a method known as salting out can concentrate the proteins from this lysate. Various types of chromatography are then used to isolate the protein or proteins of interest based on properties such as molecular weight, net charge and binding affinity.[26]: 21–24 The level of purification can be monitored using various types of gel electrophoresis if the desired protein’s molecular weight and isoelectric point are known, by spectroscopy if the protein has distinguishable spectroscopic features, or by enzyme assays if the protein has enzymatic activity. Additionally, proteins can be isolated according to their charge using electrofocusing.[56]
For natural proteins, a series of purification steps may be necessary to obtain protein sufficiently pure for laboratory applications. To simplify this process, genetic engineering is often used to add chemical features to proteins that make them easier to purify without affecting their structure or activity. Here, a «tag» consisting of a specific amino acid sequence, often a series of histidine residues (a «His-tag»), is attached to one terminus of the protein. As a result, when the lysate is passed over a chromatography column containing nickel, the histidine residues ligate the nickel and attach to the column while the untagged components of the lysate pass unimpeded. A number of different tags have been developed to help researchers purify specific proteins from complex mixtures.[57]
Cellular localization
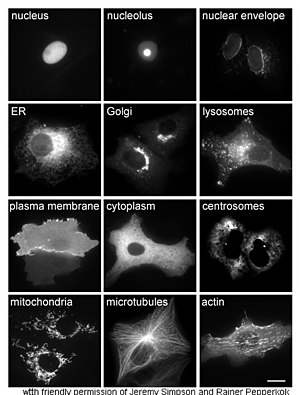
The study of proteins in vivo is often concerned with the synthesis and localization of the protein within the cell. Although many intracellular proteins are synthesized in the cytoplasm and membrane-bound or secreted proteins in the endoplasmic reticulum, the specifics of how proteins are targeted to specific organelles or cellular structures is often unclear. A useful technique for assessing cellular localization uses genetic engineering to express in a cell a fusion protein or chimera consisting of the natural protein of interest linked to a «reporter» such as green fluorescent protein (GFP).[58] The fused protein’s position within the cell can be cleanly and efficiently visualized using microscopy,[59] as shown in the figure opposite.
Other methods for elucidating the cellular location of proteins requires the use of known compartmental markers for regions such as the ER, the Golgi, lysosomes or vacuoles, mitochondria, chloroplasts, plasma membrane, etc. With the use of fluorescently tagged versions of these markers or of antibodies to known markers, it becomes much simpler to identify the localization of a protein of interest. For example, indirect immunofluorescence will allow for fluorescence colocalization and demonstration of location. Fluorescent dyes are used to label cellular compartments for a similar purpose.[60]
Other possibilities exist, as well. For example, immunohistochemistry usually uses an antibody to one or more proteins of interest that are conjugated to enzymes yielding either luminescent or chromogenic signals that can be compared between samples, allowing for localization information. Another applicable technique is cofractionation in sucrose (or other material) gradients using isopycnic centrifugation.[61] While this technique does not prove colocalization of a compartment of known density and the protein of interest, it does increase the likelihood, and is more amenable to large-scale studies.
Finally, the gold-standard method of cellular localization is immunoelectron microscopy. This technique also uses an antibody to the protein of interest, along with classical electron microscopy techniques. The sample is prepared for normal electron microscopic examination, and then treated with an antibody to the protein of interest that is conjugated to an extremely electro-dense material, usually gold. This allows for the localization of both ultrastructural details as well as the protein of interest.[62]
Through another genetic engineering application known as site-directed mutagenesis, researchers can alter the protein sequence and hence its structure, cellular localization, and susceptibility to regulation. This technique even allows the incorporation of unnatural amino acids into proteins, using modified tRNAs,[63] and may allow the rational design of new proteins with novel properties.[64]
Proteomics
The total complement of proteins present at a time in a cell or cell type is known as its proteome, and the study of such large-scale data sets defines the field of proteomics, named by analogy to the related field of genomics. Key experimental techniques in proteomics include 2D electrophoresis,[65] which allows the separation of many proteins, mass spectrometry,[66] which allows rapid high-throughput identification of proteins and sequencing of peptides (most often after in-gel digestion), protein microarrays, which allow the detection of the relative levels of the various proteins present in a cell, and two-hybrid screening, which allows the systematic exploration of protein–protein interactions.[67] The total complement of biologically possible such interactions is known as the interactome.[68] A systematic attempt to determine the structures of proteins representing every possible fold is known as structural genomics.[69]
Structure determination
Discovering the tertiary structure of a protein, or the quaternary structure of its complexes, can provide important clues about how the protein performs its function and how it can be affected, i.e. in drug design. As proteins are too small to be seen under a light microscope, other methods have to be employed to determine their structure. Common experimental methods include X-ray crystallography and NMR spectroscopy, both of which can produce structural information at atomic resolution. However, NMR experiments are able to provide information from which a subset of distances between pairs of atoms can be estimated, and the final possible conformations for a protein are determined by solving a distance geometry problem. Dual polarisation interferometry is a quantitative analytical method for measuring the overall protein conformation and conformational changes due to interactions or other stimulus. Circular dichroism is another laboratory technique for determining internal β-sheet / α-helical composition of proteins. Cryoelectron microscopy is used to produce lower-resolution structural information about very large protein complexes, including assembled viruses;[29]: 340–41 a variant known as electron crystallography can also produce high-resolution information in some cases, especially for two-dimensional crystals of membrane proteins.[70] Solved structures are usually deposited in the Protein Data Bank (PDB), a freely available resource from which structural data about thousands of proteins can be obtained in the form of Cartesian coordinates for each atom in the protein.[71]
Many more gene sequences are known than protein structures. Further, the set of solved structures is biased toward proteins that can be easily subjected to the conditions required in X-ray crystallography, one of the major structure determination methods. In particular, globular proteins are comparatively easy to crystallize in preparation for X-ray crystallography. Membrane proteins and large protein complexes, by contrast, are difficult to crystallize and are underrepresented in the PDB.[72] Structural genomics initiatives have attempted to remedy these deficiencies by systematically solving representative structures of major fold classes. Protein structure prediction methods attempt to provide a means of generating a plausible structure for proteins whose structures have not been experimentally determined.[73]
Structure prediction
Constituent amino-acids can be analyzed to predict secondary, tertiary and quaternary protein structure, in this case hemoglobin containing heme units
Complementary to the field of structural genomics, protein structure prediction develops efficient mathematical models of proteins to computationally predict the molecular formations in theory, instead of detecting structures with laboratory observation.[74] The most successful type of structure prediction, known as homology modeling, relies on the existence of a «template» structure with sequence similarity to the protein being modeled; structural genomics’ goal is to provide sufficient representation in solved structures to model most of those that remain.[75] Although producing accurate models remains a challenge when only distantly related template structures are available, it has been suggested that sequence alignment is the bottleneck in this process, as quite accurate models can be produced if a «perfect» sequence alignment is known.[76] Many structure prediction methods have served to inform the emerging field of protein engineering, in which novel protein folds have already been designed.[77] Also proteins (in eukaryotes ~33%) contain large unstructured but biologically functional segments and can be classified as intrinsically disordered proteins.[78] Predicting and analysing protein disorder is, therefore, an important part of protein structure characterisation.[79]
Bioinformatics
A vast array of computational methods have been developed to analyze the structure, function and evolution of proteins. The development of such tools has been driven by the large amount of genomic and proteomic data available for a variety of organisms, including the human genome. It is simply impossible to study all proteins experimentally, hence only a few are subjected to laboratory experiments while computational tools are used to extrapolate to similar proteins. Such homologous proteins can be efficiently identified in distantly related organisms by sequence alignment. Genome and gene sequences can be searched by a variety of tools for certain properties. Sequence profiling tools can find restriction enzyme sites, open reading frames in nucleotide sequences, and predict secondary structures. Phylogenetic trees can be constructed and evolutionary hypotheses developed using special software like ClustalW regarding the ancestry of modern organisms and the genes they express. The field of bioinformatics is now indispensable for the analysis of genes and proteins.
In silico simulation of dynamical processes
A more complex computational problem is the prediction of intermolecular interactions, such as in molecular docking,[80] protein folding, protein–protein interaction and chemical reactivity. Mathematical models to simulate these dynamical processes involve molecular mechanics, in particular, molecular dynamics. In this regard, in silico simulations discovered the folding of small α-helical protein domains such as the villin headpiece,[81] the HIV accessory protein[82] and hybrid methods combining standard molecular dynamics with quantum mechanical mathematics have explored the electronic states of rhodopsins.[83]
Beyond classical molecular dynamics, quantum dynamics methods allow the simulation of proteins in atomistic detail with an accurate description of quantum mechanical effects. Examples include the multi-layer multi-configuration time-dependent Hartree (MCTDH) method and the hierarchical equations of motion (HEOM) approach, which have been applied to plant cryptochromes[84] and bacteria light-harvesting complexes,[85] respectively. Both quantum and classical mechanical simulations of biological-scale systems are extremely computationally demanding, so distributed computing initiatives (for example, the Folding@home project[86]) facilitate the molecular modeling by exploiting advances in GPU parallel processing and Monte Carlo techniques.
Chemical analysis
The total nitrogen content of organic matter is mainly formed by the amino groups in proteins. The Total Kjeldahl Nitrogen (TKN) is a measure of nitrogen widely used in the analysis of (waste) water, soil, food, feed and organic matter in general. As the name suggests, the Kjeldahl method is applied. More sensitive methods are available.[87][88]
Nutrition
Most microorganisms and plants can biosynthesize all 20 standard amino acids, while animals (including humans) must obtain some of the amino acids from the diet.[43] The amino acids that an organism cannot synthesize on its own are referred to as essential amino acids. Key enzymes that synthesize certain amino acids are not present in animals—such as aspartokinase, which catalyses the first step in the synthesis of lysine, methionine, and threonine from aspartate. If amino acids are present in the environment, microorganisms can conserve energy by taking up the amino acids from their surroundings and downregulating their biosynthetic pathways.
In animals, amino acids are obtained through the consumption of foods containing protein. Ingested proteins are then broken down into amino acids through digestion, which typically involves denaturation of the protein through exposure to acid and hydrolysis by enzymes called proteases. Some ingested amino acids are used for protein biosynthesis, while others are converted to glucose through gluconeogenesis, or fed into the citric acid cycle. This use of protein as a fuel is particularly important under starvation conditions as it allows the body’s own proteins to be used to support life, particularly those found in muscle.[89]
In animals such as dogs and cats, protein maintains the health and quality of the skin by promoting hair follicle growth and keratinization, and thus reducing the likelihood of skin problems producing malodours.[90] Poor-quality proteins also have a role regarding gastrointestinal health, increasing the potential for flatulence and odorous compounds in dogs because when proteins reach the colon in an undigested state, they are fermented producing hydrogen sulfide gas, indole, and skatole.[91] Dogs and cats digest animal proteins better than those from plants, but products of low-quality animal origin are poorly digested, including skin, feathers, and connective tissue.[91]
See also
- Deproteination
- DNA-binding protein
- Macromolecule
- Index of protein-related articles
- Intein
- List of proteins
- Proteopathy
- Proteopedia
- Proteolysis
- Protein sequence space
- Protein superfamily
References
- ^ Thomas Burr Osborne (1909): The Vegetable Proteins Archived 2016-03-22 at the Wayback Machine, History pp 1 to 6, from archive.org
- ^ Mulder GJ (1838). «Sur la composition de quelques substances animales». Bulletin des Sciences Physiques et Naturelles en Néerlande: 104.
- ^ Harold H (1951). «Origin of the Word ‘Protein.’«. Nature. 168 (4267): 244. Bibcode:1951Natur.168..244H. doi:10.1038/168244a0. PMID 14875059. S2CID 4271525.
- ^ a b c Perrett D (August 2007). «From ‘protein’ to the beginnings of clinical proteomics». Proteomics: Clinical Applications. 1 (8): 720–38. doi:10.1002/prca.200700525. PMID 21136729. S2CID 32843102.
- ^ New Oxford Dictionary of English
- ^ Reynolds JA, Tanford C (2003). Nature’s Robots: A History of Proteins (Oxford Paperbacks). New York, New York: Oxford University Press. p. 15. ISBN 978-0-19-860694-9.
- ^ Reynolds and Tanford (2003).
- ^ Bischoff TL, Voit C (1860). Die Gesetze der Ernaehrung des Pflanzenfressers durch neue Untersuchungen festgestellt (in German). Leipzig, Heidelberg.
- ^ «Hofmeister, Franz». encyclopedia.com. Archived from the original on 5 April 2017. Retrieved 4 April 2017.
- ^ «Protein, section: Classification of protein». britannica.com. Archived from the original on 4 April 2017. Retrieved 4 April 2017.
- ^ Sumner JB (1926). «The isolation and crystallization of the enzyme urease. Preliminary paper» (PDF). Journal of Biological Chemistry. 69 (2): 435–41. doi:10.1016/S0021-9258(18)84560-4. Archived from the original on 2011-03-25. Retrieved 2011-01-16.
- ^ Pauling L, Corey RB (May 1951). «Atomic coordinates and structure factors for two helical configurations of polypeptide chains» (PDF). Proceedings of the National Academy of Sciences of the United States of America. 37 (5): 235–40. Bibcode:1951PNAS…37..235P. doi:10.1073/pnas.37.5.235. PMC 1063348. PMID 14834145. Archived (PDF) from the original on 2012-11-28. Retrieved 2009-04-14.
- ^ Kauzmann W (May 1956). «Structural factors in protein denaturation». Journal of Cellular Physiology. 47 (Suppl 1): 113–31. doi:10.1002/jcp.1030470410. PMID 13332017.
- ^ Kauzmann W (1959). «Some factors in the interpretation of protein denaturation». Advances in Protein Chemistry Volume 14. Advances in Protein Chemistry. Vol. 14. pp. 1–63. doi:10.1016/S0065-3233(08)60608-7. ISBN 978-0-12-034214-3. PMID 14404936.
- ^ Kalman SM, Linderstrøm-Lang K, Ottesen M, Richards FM (February 1955). «Degradation of ribonuclease by subtilisin». Biochimica et Biophysica Acta. 16 (2): 297–99. doi:10.1016/0006-3002(55)90224-9. PMID 14363272.
- ^ Sanger F (1949). «The terminal peptides of insulin». The Biochemical Journal. 45 (5): 563–74. doi:10.1042/bj0450563. PMC 1275055. PMID 15396627.
- ^ Sanger F. (1958), Nobel lecture: The chemistry of insulin (PDF), Nobelprize.org, archived (PDF) from the original on 2013-03-19, retrieved 2016-02-09
- ^ a b c Stoddart, Charlotte (1 March 2022). «Structural biology: How proteins got their close-up». Knowable Magazine. doi:10.1146/knowable-022822-1. Retrieved 25 March 2022.
- ^ Muirhead H, Perutz MF (August 1963). «Structure of hemoglobin. A three-dimensional fourier synthesis of reduced human hemoglobin at 5.5 Å resolution». Nature. 199 (4894): 633–38. Bibcode:1963Natur.199..633M. doi:10.1038/199633a0. PMID 14074546. S2CID 4257461.
- ^ Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC (March 1958). «A three-dimensional model of the myoglobin molecule obtained by x-ray analysis». Nature. 181 (4610): 662–66. Bibcode:1958Natur.181..662K. doi:10.1038/181662a0. PMID 13517261. S2CID 4162786.
- ^ Zhou ZH (April 2008). «Towards atomic resolution structural determination by single-particle cryo-electron microscopy». Current Opinion in Structural Biology. 18 (2): 218–28. doi:10.1016/j.sbi.2008.03.004. PMC 2714865. PMID 18403197.
- ^ Keskin O, Tuncbag N, Gursoy A (April 2008). «Characterization and prediction of protein interfaces to infer protein-protein interaction networks». Current Pharmaceutical Biotechnology. 9 (2): 67–76. doi:10.2174/138920108783955191. hdl:11511/32640. PMID 18393863.
- ^ «RCSB Protein Data Bank». Archived from the original on 2015-04-18. Retrieved 2017-01-19.
- ^ Nelson DL, Cox MM (2005). Lehninger’s Principles of Biochemistry (4th ed.). New York, New York: W. H. Freeman and Company.
- ^ Gutteridge A, Thornton JM (November 2005). «Understanding nature’s catalytic toolkit». Trends in Biochemical Sciences. 30 (11): 622–29. doi:10.1016/j.tibs.2005.09.006. PMID 16214343.
- ^ a b c d e f g Murray RF, Harper HW, Granner DK, Mayes PA, Rodwell VW (2006). Harper’s Illustrated Biochemistry. New York: Lange Medical Books/McGraw-Hill. ISBN 978-0-07-146197-9.
- ^ a b c Lodish H, Berk A, Matsudaira P, Kaiser CA, Krieger M, Scott MP, Zipurksy SL, Darnell J (2004). Molecular Cell Biology (5th ed.). New York, New York: WH Freeman and Company.
- ^ Ardejani MS, Powers ET, Kelly JW (August 2017). «Using Cooperatively Folded Peptides To Measure Interaction Energies and Conformational Propensities». Accounts of Chemical Research. 50 (8): 1875–1882. doi:10.1021/acs.accounts.7b00195. PMC 5584629. PMID 28723063.
- ^ a b c d Branden C, Tooze J (1999). Introduction to Protein Structure. New York: Garland Pub. ISBN 978-0-8153-2305-1.
- ^ a b c d e f g h i j Van Holde KE, Mathews CK (1996). Biochemistry. Menlo Park, California: Benjamin/Cummings Pub. Co., Inc. ISBN 978-0-8053-3931-4.
- ^ Milo R (December 2013). «What is the total number of protein molecules per cell volume? A call to rethink some published values». BioEssays. 35 (12): 1050–55. doi:10.1002/bies.201300066. PMC 3910158. PMID 24114984.
- ^ Beck M, Schmidt A, Malmstroem J, Claassen M, Ori A, Szymborska A, Herzog F, Rinner O, Ellenberg J, Aebersold R (November 2011). «The quantitative proteome of a human cell line». Molecular Systems Biology. 7: 549. doi:10.1038/msb.2011.82. PMC 3261713. PMID 22068332.
- ^ Wu L, Candille SI, Choi Y, Xie D, Jiang L, Li-Pook-Than J, Tang H, Snyder M (July 2013). «Variation and genetic control of protein abundance in humans». Nature. 499 (7456): 79–82. Bibcode:2013Natur.499…79W. doi:10.1038/nature12223. PMC 3789121. PMID 23676674.
- ^ Dobson CM (2000). «The nature and significance of protein folding». In Pain RH (ed.). Mechanisms of Protein Folding. Oxford, Oxfordshire: Oxford University Press. pp. 1–28. ISBN 978-0-19-963789-8.
- ^ Kozlowski LP (January 2017). «Proteome-pI: proteome isoelectric point database». Nucleic Acids Research. 45 (D1): D1112–D1116. doi:10.1093/nar/gkw978. PMC 5210655. PMID 27789699.
- ^ Fulton AB, Isaacs WB (April 1991). «Titin, a huge, elastic sarcomeric protein with a probable role in morphogenesis». BioEssays. 13 (4): 157–61. doi:10.1002/bies.950130403. PMID 1859393. S2CID 20237314.
- ^ Bruckdorfer T, Marder O, Albericio F (February 2004). «From production of peptides in milligram amounts for research to multi-tons quantities for drugs of the future». Current Pharmaceutical Biotechnology. 5 (1): 29–43. doi:10.2174/1389201043489620. PMID 14965208.
- ^ Schwarzer D, Cole PA (December 2005). «Protein semisynthesis and expressed protein ligation: chasing a protein’s tail». Current Opinion in Chemical Biology. 9 (6): 561–69. doi:10.1016/j.cbpa.2005.09.018. PMID 16226484.
- ^ Kent SB (February 2009). «Total chemical synthesis of proteins». Chemical Society Reviews. 38 (2): 338–51. doi:10.1039/b700141j. PMID 19169452. S2CID 5432012.
- ^ Fernández A, Scott R (September 2003). «Dehydron: a structurally encoded signal for protein interaction». Biophysical Journal. 85 (3): 1914–28. Bibcode:2003BpJ….85.1914F. doi:10.1016/S0006-3495(03)74619-0. PMC 1303363. PMID 12944304.
- ^ Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, Budd A, Diella F, Dinkel H, Gibson TJ (January 2012). «Attributes of short linear motifs». Molecular BioSystems. 8 (1): 268–81. doi:10.1039/c1mb05231d. PMID 21909575.
- ^ [Scalvini B, Sheikhhassani V, Woodard J, Aupič J, Dame RT, Jerala R, Mashaghi A, Topology of Folded Molecular Chains: From Single Biomolecules to Engineered Origami. Trends in Chemistry 2(7), P609-622 (2020) https://www.sciencedirect.com/science/article/pii/S2589597420301118]
- ^ a b Voet D, Voet JG. (2004). Biochemistry Vol 1 3rd ed. Wiley: Hoboken, NJ.
- ^ Sankaranarayanan R, Moras D (2001). «The fidelity of the translation of the genetic code». Acta Biochimica Polonica. 48 (2): 323–35. doi:10.18388/abp.2001_3918. PMID 11732604.
- ^ Copland JA, Sheffield-Moore M, Koldzic-Zivanovic N, Gentry S, Lamprou G, Tzortzatou-Stathopoulou F, Zoumpourlis V, Urban RJ, Vlahopoulos SA (June 2009). «Sex steroid receptors in skeletal differentiation and epithelial neoplasia: is tissue-specific intervention possible?». BioEssays. 31 (6): 629–41. doi:10.1002/bies.200800138. PMID 19382224. S2CID 205469320.
- ^ Samarin S, Nusrat A (January 2009). «Regulation of epithelial apical junctional complex by Rho family GTPases». Frontiers in Bioscience. 14 (14): 1129–42. doi:10.2741/3298. PMID 19273120.
- ^ Bairoch A (January 2000). «The ENZYME database in 2000» (PDF). Nucleic Acids Research. 28 (1): 304–05. doi:10.1093/nar/28.1.304. PMC 102465. PMID 10592255. Archived from the original (PDF) on June 1, 2011.
- ^ Radzicka A, Wolfenden R (January 1995). «A proficient enzyme». Science. 267 (5194): 90–3. Bibcode:1995Sci…267…90R. doi:10.1126/science.7809611. PMID 7809611.
- ^ EBI External Services (2010-01-20). «The Catalytic Site Atlas at The European Bioinformatics Institute». Ebi.ac.uk. Archived from the original on 2013-08-03. Retrieved 2011-01-16.
- ^ Pickel B, Schaller A (October 2013). «Dirigent proteins: molecular characteristics and potential biotechnological applications». Applied Microbiology and Biotechnology. 97 (19): 8427–38. doi:10.1007/s00253-013-5167-4. PMID 23989917. S2CID 1896003.
- ^ Rüdiger H, Siebert HC, Solís D, Jiménez-Barbero J, Romero A, von der Lieth CW, Diaz-Mariño T, Gabius HJ (April 2000). «Medicinal chemistry based on the sugar code: fundamentals of lectinology and experimental strategies with lectins as targets». Current Medicinal Chemistry. 7 (4): 389–416. doi:10.2174/0929867003375164. PMID 10702616.
- ^ Mulder NJ (2007-09-28). «Protein Family Databases». eLS. Chichester, UK: John Wiley & Sons, Ltd. pp. a0003058.pub2. doi:10.1002/9780470015902.a0003058.pub2. ISBN 978-0-470-01617-6.
- ^ Sisu C, Pei B, Leng J, Frankish A, Zhang Y, Balasubramanian S, et al. (September 2014). «Comparative analysis of pseudogenes across three phyla». Proceedings of the National Academy of Sciences of the United States of America. 111 (37): 13361–6. Bibcode:2014PNAS..11113361S. doi:10.1073/pnas.1407293111. PMC 4169933. PMID 25157146.
- ^ a b Guzmán GI, Sandberg TE, LaCroix RA, Nyerges Á, Papp H, de Raad M, et al. (April 2019). «Enzyme promiscuity shapes adaptation to novel growth substrates». Molecular Systems Biology. 15 (4): e8462. doi:10.15252/msb.20188462. PMC 6452873. PMID 30962359.
- ^ Roohi, Bano K, Kuddus M, Zaheer MR, Zia Q, Khan MF, Ashraf GM, Gupta A, Aliev G (2017). «Microbial Enzymatic Degradation of Biodegradable Plastics». Current Pharmaceutical Biotechnology. 18 (5): 429–440. doi:10.2174/1389201018666170523165742. PMID 28545359.
- ^ Hey J, Posch A, Cohen A, Liu N, Harbers A (2008). «Fractionation of complex protein mixtures by liquid-phase isoelectric focusing». 2D PAGE: Sample Preparation and Fractionation. Methods in Molecular Biology. Vol. 424. pp. 225–39. doi:10.1007/978-1-60327-064-9_19. ISBN 978-1-58829-722-8. PMID 18369866.
- ^ Terpe K (January 2003). «Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems». Applied Microbiology and Biotechnology. 60 (5): 523–33. doi:10.1007/s00253-002-1158-6. PMID 12536251. S2CID 206934268.
- ^ Stepanenko OV, Verkhusha VV, Kuznetsova IM, Uversky VN, Turoverov KK (August 2008). «Fluorescent proteins as biomarkers and biosensors: throwing color lights on molecular and cellular processes». Current Protein & Peptide Science. 9 (4): 338–69. doi:10.2174/138920308785132668. PMC 2904242. PMID 18691124.
- ^ Yuste R (December 2005). «Fluorescence microscopy today». Nature Methods. 2 (12): 902–4. doi:10.1038/nmeth1205-902. PMID 16299474. S2CID 205418407.
- ^ Margolin W (January 2000). «Green fluorescent protein as a reporter for macromolecular localization in bacterial cells». Methods. 20 (1): 62–72. doi:10.1006/meth.1999.0906. PMID 10610805.
- ^ Walker JH, Wilson K (2000). Principles and Techniques of Practical Biochemistry. Cambridge, UK: Cambridge University Press. pp. 287–89. ISBN 978-0-521-65873-7.
- ^ Mayhew TM, Lucocq JM (August 2008). «Developments in cell biology for quantitative immunoelectron microscopy based on thin sections: a review». Histochemistry and Cell Biology. 130 (2): 299–313. doi:10.1007/s00418-008-0451-6. PMC 2491712. PMID 18553098.
- ^ Hohsaka T, Sisido M (December 2002). «Incorporation of non-natural amino acids into proteins». Current Opinion in Chemical Biology. 6 (6): 809–15. doi:10.1016/S1367-5931(02)00376-9. PMID 12470735.
- ^ Cedrone F, Ménez A, Quéméneur E (August 2000). «Tailoring new enzyme functions by rational redesign». Current Opinion in Structural Biology. 10 (4): 405–10. doi:10.1016/S0959-440X(00)00106-8. PMID 10981626.
- ^ Görg A, Weiss W, Dunn MJ (December 2004). «Current two-dimensional electrophoresis technology for proteomics». Proteomics. 4 (12): 3665–85. doi:10.1002/pmic.200401031. PMID 15543535. S2CID 28594824.
- ^ Conrotto P, Souchelnytskyi S (September 2008). «Proteomic approaches in biological and medical sciences: principles and applications». Experimental Oncology. 30 (3): 171–80. PMID 18806738.
- ^ Koegl M, Uetz P (December 2007). «Improving yeast two-hybrid screening systems». Briefings in Functional Genomics & Proteomics. 6 (4): 302–12. doi:10.1093/bfgp/elm035. PMID 18218650. Archived from the original on 2017-09-11. Retrieved 2017-07-23.
- ^ Plewczyński D, Ginalski K (2009). «The interactome: predicting the protein-protein interactions in cells». Cellular & Molecular Biology Letters. 14 (1): 1–22. doi:10.2478/s11658-008-0024-7. PMC 6275871. PMID 18839074.
- ^ Zhang C, Kim SH (February 2003). «Overview of structural genomics: from structure to function». Current Opinion in Chemical Biology. 7 (1): 28–32. doi:10.1016/S1367-5931(02)00015-7. PMID 12547423. Archived from the original on 2018-11-19. Retrieved 2019-06-29.
- ^ Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T (December 2005). «Lipid-protein interactions in double-layered two-dimensional AQP0 crystals». Nature. 438 (7068): 633–38. Bibcode:2005Natur.438..633G. doi:10.1038/nature04321. PMC 1350984. PMID 16319884.
- ^ Standley DM, Kinjo AR, Kinoshita K, Nakamura H (July 2008). «Protein structure databases with new web services for structural biology and biomedical research». Briefings in Bioinformatics. 9 (4): 276–85. doi:10.1093/bib/bbn015. PMID 18430752.
- ^ Walian P, Cross TA, Jap BK (2004). «Structural genomics of membrane proteins». Genome Biology. 5 (4): 215. doi:10.1186/gb-2004-5-4-215. PMC 395774. PMID 15059248.
- ^ Sleator RD (2012). «Prediction of protein functions». Functional Genomics. Methods in Molecular Biology. Vol. 815. pp. 15–24. doi:10.1007/978-1-61779-424-7_2. ISBN 978-1-61779-423-0. PMID 22130980.
- ^ Zhang Y (June 2008). «Progress and challenges in protein structure prediction». Current Opinion in Structural Biology. 18 (3): 342–48. doi:10.1016/j.sbi.2008.02.004. PMC 2680823. PMID 18436442.
- ^ Xiang Z (June 2006). «Advances in homology protein structure modeling». Current Protein & Peptide Science. 7 (3): 217–27. doi:10.2174/138920306777452312. PMC 1839925. PMID 16787261.
- ^ Zhang Y, Skolnick J (January 2005). «The protein structure prediction problem could be solved using the current PDB library». Proceedings of the National Academy of Sciences of the United States of America. 102 (4): 1029–34. Bibcode:2005PNAS..102.1029Z. doi:10.1073/pnas.0407152101. PMC 545829. PMID 15653774.
- ^ Kuhlman B, Dantas G, Ireton GC, Varani G, Stoddard BL, Baker D (November 2003). «Design of a novel globular protein fold with atomic-level accuracy». Science. 302 (5649): 1364–68. Bibcode:2003Sci…302.1364K. doi:10.1126/science.1089427. PMID 14631033. S2CID 1939390.
- ^ Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT (March 2004). «Prediction and functional analysis of native disorder in proteins from the three kingdoms of life». Journal of Molecular Biology. 337 (3): 635–45. CiteSeerX 10.1.1.120.5605. doi:10.1016/j.jmb.2004.02.002. PMID 15019783.
- ^ Tompa P, Fersht A (18 November 2009). Structure and Function of Intrinsically Disordered Proteins. CRC Press. ISBN 978-1-4200-7893-0. Archived from the original on 19 April 2017. Retrieved 19 October 2016.
- ^ Ritchie DW (February 2008). «Recent progress and future directions in protein-protein docking». Current Protein & Peptide Science. 9 (1): 1–15. CiteSeerX 10.1.1.211.4946. doi:10.2174/138920308783565741. PMID 18336319.
- ^ Zagrovic B, Snow CD, Shirts MR, Pande VS (November 2002). «Simulation of folding of a small alpha-helical protein in atomistic detail using worldwide-distributed computing». Journal of Molecular Biology. 323 (5): 927–37. CiteSeerX 10.1.1.142.8664. doi:10.1016/S0022-2836(02)00997-X. PMID 12417204.
- ^ Herges T, Wenzel W (January 2005). «In silico folding of a three helix protein and characterization of its free-energy landscape in an all-atom force field». Physical Review Letters. 94 (1): 018101. arXiv:physics/0310146. Bibcode:2005PhRvL..94a8101H. doi:10.1103/PhysRevLett.94.018101. PMID 15698135. S2CID 1477100.
- ^ Hoffmann M, Wanko M, Strodel P, König PH, Frauenheim T, Schulten K, Thiel W, Tajkhorshid E, Elstner M (August 2006). «Color tuning in rhodopsins: the mechanism for the spectral shift between bacteriorhodopsin and sensory rhodopsin II». Journal of the American Chemical Society. 128 (33): 10808–18. doi:10.1021/ja062082i. PMID 16910676.
- ^ Mendive-Tapia D, Mangaud E, Firmino T, de la Lande A, Desouter-Lecomte M, Meyer HD, Gatti F (2018). «Multidimensional Quantum Mechanical Modeling of Electron Transfer and Electronic Coherence in Plant Cryptochromes: The Role of Initial Bath Conditions». J. Phys. Chem. B. 122 (1): 126–136. doi:10.1021/acs.jpcb.7b10412. PMID 29216421.
- ^ Strümpfer J, Schulten K (2012). «Open Quantum Dynamics Calculations with the Hierarchy Equations of Motion on Parallel Computers». J. Chem. Theory Comput. 8 (8): 2808–2816. doi:10.1021/ct3003833. PMC 3480185. PMID 23105920.
- ^ Scheraga HA, Khalili M, Liwo A (2007). «Protein-folding dynamics: overview of molecular simulation techniques». Annual Review of Physical Chemistry. 58: 57–83. Bibcode:2007ARPC…58…57S. doi:10.1146/annurev.physchem.58.032806.104614. PMID 17034338.
- ^ Muñoz-Huerta, Rafael F.; Guevara-Gonzalez, Ramon G.; Contreras-Medina, Luis M.; Torres-Pacheco, Irineo; Prado-Olivarez, Juan; Ocampo-Velazquez, Rosalia V. (Aug 16, 2013). «A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances». Sensors (Basel, Switzerland). 13 (8): 10823–10843. Bibcode:2013Senso..1310823M. doi:10.3390/s130810823. PMC 3812630. PMID 23959242.
- ^ Martin, P D; Malley, D F; Manning, G.; Fuller, L. (Nov 1, 2002). «Determination of soil organic carbon and nitrogen at the field level using near-infrared spectroscopy». Canadian Journal of Soil Science. 82 (4): 413–422. doi:10.4141/S01-054.
- ^ Brosnan JT (June 2003). «Interorgan amino acid transport and its regulation». The Journal of Nutrition. 133 (6 Suppl 1): 2068S–72S. doi:10.1093/jn/133.6.2068S. PMID 12771367.
- ^ Watson TD (1998). «Diet and skin disease in dogs and cats». The Journal of Nutrition. 128 (12 Suppl): 2783S–89S. doi:10.1093/jn/128.12.2783S. PMID 9868266.
- ^ a b Case LP, Daristotle L, Hayek MG, Raasch MF (2010). Canine and Feline Nutrition-E-Book: A Resource for Companion Animal Professionals. Elsevier Health Sciences.
Further reading
- Textbooks
- Branden C, Tooze J (1999). Introduction to Protein Structure. New York: Garland Pub. ISBN 978-0-8153-2305-1.
- Murray RF, Harper HW, Granner DK, Mayes PA, Rodwell VW (2006). Harper’s Illustrated Biochemistry. New York: Lange Medical Books/McGraw-Hill. ISBN 978-0-07-146197-9.
- Van Holde KE, Mathews CK (1996). Biochemistry. Menlo Park, California: Benjamin/Cummings Pub. Co., Inc. ISBN 978-0-8053-3931-4.
External links
Wikimedia Commons has media related to Proteins.
Look up protein in Wiktionary, the free dictionary.
Databases and projects
- NCBI Entrez Protein database
- NCBI Protein Structure database
- Human Protein Reference Database
- Human Proteinpedia
- Folding@Home (Stanford University) Archived 2012-09-08 at the Wayback Machine
- Protein Databank in Europe (see also PDBeQuips[permanent dead link], short articles and tutorials on interesting PDB structures)
- Research Collaboratory for Structural Bioinformatics (see also Molecule of the Month Archived 2020-07-24 at the Wayback Machine, presenting short accounts on selected proteins from the PDB)
- Proteopedia – Life in 3D: rotatable, zoomable 3D model with wiki annotations for every known protein molecular structure.
- UniProt the Universal Protein Resource
Tutorials and educational websites
- «An Introduction to Proteins» from HOPES (Huntington’s Disease Outreach Project for Education at Stanford)
- Proteins: Biogenesis to Degradation – The Virtual Library of Biochemistry and Cell Biology
The origin of the word protein comes from the Greek «Proteos» which means first or fundamental. Proteins are macromolecules (very large molecules), which are formed or are born from the union of other types of molecules, which are called amino acids. These large molecules are the main source of nutrition so that the muscles of the body can be formed in the best way, they also have the property of regenerating and forming new cells and transporting oxygen.
These macromolecules are formed from a linear structure of amino acid molecules that have the ability to join through peptide bonds. Each type of protein has specific functions, for example some serve as transport, that is, they are responsible for carrying various substances to the blood, such as hemoglobin, which transfers oxygen to the tissues and is also responsible for collecting carbon dioxide. carbon to carry it to the lungs so that it can be eliminated. Another is the case of proteins that are responsible for genetics, establishing DNA replication. There are defensive proteins such as antibodies, there are also regulatory proteins such as insulin necessary to regulate theglycemia or level of sugar that is present in the blood. On the other hand there are the catalysts that allow some biochemical process, such as digestive enzymes that help the body to obtain from them the various nutrients it needs through the digestion process.
There is a classification of proteins depending on the physical and chemical properties that they present, in the first place there are the simple proteins also called holoproteids, which have a composition only of amino acids, on the other hand there are conjugated or heteroprotein proteins, apart from being made up of amino acids, it also has the presence of various substances and finally there are derived proteins that are formed by denaturation or splitting of some other compound.
The body obtains the proteins it needs through food, it is important to note that different types of food provide different types of protein, the consumption of dairy products, vegetables, meats and legumes provide different proteins useful for the development of the body
A representation of the three-dimensional structure of myoglobin, the oxygen carrier in muscle. Max Perutz and Sir John Cowdery Kendrew received a Nobel Prize in Chemistry for their elucidation of myoglobin’s structure in 1958; it was the first protein whose structure was solved using X-ray crystallography. The colored alpha helices represent myoglobin’s secondary structure (discussed below).
A protein is a biological polymer comprising numerous amino acids linked recursively through peptide bonds between a carboxyl group and an amino group of adjacent amino acids to form a long chain with the defining side group of each amino acid protruding from it. The sequence of amino acids in a protein is defined by a gene and encoded in the genetic code, which selects protein components from a set of 20 «standard» amino acids.
Some proteins function as separate entities while others associate together to form stable functional complexes, such as the ribosomes, which comprise more than 50 proteins. Along with polysaccharides, lipids, and nucleic acids, proteins are one of the major classes of macromolecules that make up the primary constituents of biological organisms.
As suggested by the etymological origins of the term (from the Greek word proteios, meaning “of the first order”), proteins are of prime importance in the structure and function of all living cells and viruses. Different proteins perform a wide variety of biological functions. Some proteins are enzymes, catalyzing the chemical reactions in an organism. Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton, which is like a system of scaffolding within a cell. Still others, such as antibodies, are able to identify and neutralize foreign substances like bacteria and viruses.
Dietary protein is essential for the survival of animals. Unlike plants, which are able to synthesize all the amino acids they require, animals can only synthesize some of the 20 standard amino acids necessary for normal functioning. The amino acids required in the animal diet are known as essential amino acids, though their specific number and type vary among species.
The functionality of a protein is dependent upon its ability to fold into a precise three-dimensional shape. This complex folding remains a mystery and reveals a remarkable complexity and harmony in our universe. As Lewis (2005) notes, «there are so many solutions it would not be possible for a protein to test all of these until it finds the right one, it would take too long. A small chain of 150 amino acids testing 1012 different configurations each second would take about 1026 years—a billion, billion times the age of the universe—to find the ‘correct configuration.’ Yet, the refolding of a denatured enzyme takes place in less than a minute.»
Discovered by Jöns Jakob Berzelius in 1838, proteins are among the most actively studied molecules in biochemistry. Biochemists are interested in determining a protein’s unique amino acid sequence, which is presumed to govern its three-dimensional structure and, in turn, its biological function. Knowing a protein’s amino acid sequence can be helpful in the study and treatment of disease, since a change in a single amino acid in a single protein (which often reflects a mutation in a particular gene) can result in diseases such as sickle-cell anemia and cystic fibrosis. Charting the amino acid sequences of proteins contributes to a reconstruction of the history of early life, as proteins resemble one another in sequence only if they evolved from a common ancestor.
The structure of proteins
Components and synthesis
Proteins are built from combinations of 20 different biological amino acids, which are molecules composed of a central or alpha carbon with three attachments: an amino group (-NH2), a carboxylic acid group (-COOH), and a unique R group, or side chain. In proteins, amino acids (specifically, alpha-amino acids) are linked together by peptide bonds, which form when the amino group of one amino acid reacts with the carboxyl group of a second amino acid to form a covalent bond after releasing a water molecule. An amino acid residue is what is left of an amino acid once it has coupled with another amino acid to form a peptide bond.
Proteins are generally large molecules (e.g., the muscle protein titin or connectin has a single amino acid chain that is 27,000 subunits long). Such long chains of amino acids are almost universally referred to as proteins, but shorter strings of amino acids may be referred to as polypeptides, peptides, or, less commonly, oligopeptides. The variation in protein size contributes to their functional diversity—for instance, a shorter amino acid chain may be more likely to act as a hormone (like insulin), rather than as an enzyme (which depends on its defined three-dimensional structure for functionality).
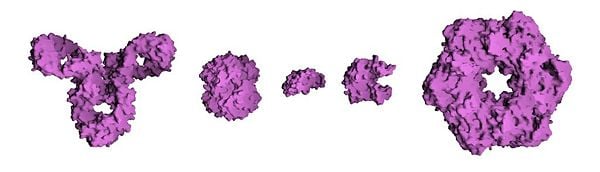
The molecular surfaces of several proteins showing their comparative sizes. From left to right: immunoglobin G (an antibody), hemoglobin (a transport protein), insulin (a hormone), adenylate kinase (an enzyme), and glutamine synthetase (an enzyme).
Proteins are assembled from amino acids based on information encoded as genes, specific nucleotide sequences in the DNA. From the DNA, the protein-coding nucleotide sequences are each transcribed into an immature messenger RNA (mRNA), which is then cleaned up and modified to form the mature mRNA that is translated into a protein. In many cases, the resulting protein is further chemically altered (post-translational modification) before it becomes functional.
The four levels of protein structure
The four levels of protein structure
Proteins fold into unique three-dimensional structures. The shape into which a protein naturally folds is known as its native state, which is presumed to be determined by its sequence of amino acids. Sometimes, however, proteins do not fold properly. The incorrect folding of proteins can lead to illnesses such as Alzheimer’s disease, in which brain function is limited by deposits of incorrectly-folded proteins that can no longer perform their functions. A full understanding of why incorrect protein folding occurs might lead to advances in the treatment of diseases like Alzheimer’s.
Biochemists refer to four distinct aspects of a protein’s structure:
- Primary structure is the linear amino acid sequence encoded by DNA. Any error in this sequence, such as the substitution of one amino acid residue for another, may lead to a congenital disease.
- Secondary structures are highly patterned sub-structures that form in the interaction of amino acid residues near to each other on the chain. The most common are the alpha helix and the beta sheet. There can be many different secondary motifs present in one single protein molecule.
- Tertiary structure refers to the overall, three-dimensional shape of a single protein molecule. This spatial relationship of amino acid residues that are far apart on the sequence is primarily formed by hydrophobic interactions, though hydrogen bonds and ionic interactions, and disulfide bonds are usually involved as well.
- Some proteins may have a quaternary structure, a shape or structure that results from the union of more than one protein molecule (called subunits in this context), which function as part of the larger assembly, or protein complex. Hemoglobin, which serves as an oxygen carrier in blood, has a quaternary structure of four subunits.
The quaternary structure of hemoglobin. The four subunits are shown in red and yellow; the iron-containing heme groups are in green.
In addition to these levels of structure, proteins may shift between several similar structures in performing their biological function. In the context of these functional rearrangements, tertiary or quaternary structures are usually referred to as conformations, and transitions between them are called conformational changes. Although any unique polypeptide may have more than one stable folded conformation, each conformation has its own biological activity, and only one conformation is considered to be the active one. This assumption has been recently challenged, however, by the discovery of intrinsically unstructured proteins, which can fold in multiple structures with different biological activity.
Major functions of proteins
The enzyme hexokinase is shown as a simple ball-and-stick molecular model. To scale in the top right-hand corner are its two substrates, ATP and glucose.
Proteins are involved in practically every function performed by a cell, including regulation of cellular functions such as signal transduction and metabolism. However, several major classes of proteins may be identified based on the functions below:
- Enzyme catalysis. Nearly all of the chemical reactions in living organisms—from the initial breakdown of food nutrients in the saliva to the replication of DNA—are catalyzed by proteins.
- Transport and storage. Membrane-associated proteins move their substrates (such as small molecules and ions) from place to place without altering their chemical properties. For example, the protein hemoglobin (pictured above) transports oxygen in blood.
- Immune protection. Antibodies, the basis of the adaptive immune system, are soluble proteins capable of recognizing and combining with foreign substances. This class also includes toxins, which play a defensive role (e.g., the dendrotoxins of snakes).
- Signaling. Receptors mediate the responses of nerve cells to specific stimuli. Rhodopsin, for example, is a light sensitive protein in the rod cells of the retina of vertebrates.
- Structural support. Examples include tubulin, actin, collagen, and keratin, which are important strengthening components of skin, hair, and bone.
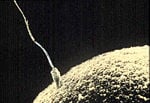
The flagella is composed of motor proteins that propel sperm cells toward the ovum for fertilization
- Coordinated motion. Another special class of proteins consists of motor proteins such as myosin, kinesin, and dynein. These proteins are «molecular motors,» generating physical force which can move organelles, cells, and entire muscles. Proteins are the major components of muscle, and muscle contraction involves the sliding motion of two kinds of protein filaments. At the microscopic level, the propulsion of sperm by flagella is produced by protein assemblies.
- Control of growth and differentiation. In higher organisms, ‘growth factor proteins such as insulin control the growth and differentiation of cells. Transcription factors regulate the activation of transcription in eukaryotes, while cyclins regulate the cell cycle, the series of events in a eukaryotic cell between one cell division and the next.
Proteins in the human diet
Sources of protein
Soybeans are a good source of essential amino acids
Protein is an important macronutrient in the human diet, supplying the body’s needs for amino acids, particularly the essential amino acids that humans are unable to synthesize. Between eight and ten amino acids are considered essential for humans.
While animal meats are rich sources of this vital dietary element, protein is also found in plant foods, such as grains and legumes, and in eggs and dairy products, such as milk and yogurt. The best way to obtain the full range of essential amino acids is to consume a variety of protein-rich foods. Soy products such as tofu are particularly important to many vegetarians and vegans as a source of complete protein (a protein that contains significant amounts of all the essential amino acids).
The exact amount of dietary protein needed to satisfy protein requirements for humans, known as a Recommended Dietary Allowance (RDA), may vary widely depending on age, sex, level of physical activity, and medical condition.
Protein deficiency and dietary imbalance
A child with kwashiorkor in Nigeria
Protein deficiency can lead to symptoms such as fatigue, insulin resistance, hair loss, loss of hair pigment, loss of muscle mass, low body temperature, hormonal irregularities, and loss of skin elasticity. Severe protein deficiency is most commonly encountered in developing countries in times of famine, when diets are high in starch and low in protein. Kwashiorkor is a type of childhood malnutrition that is linked to insufficient protein intake (and may also result from deficiencies in various nutrients), though its causes are not fully understood.
Given the central importance of proteins to life, particularly the importance of strong muscles for survival, animals are designed to minimize the loss of protein from muscle during periods of starvation. When dietary proteins and carbohydrates are deficient, proteins may be broken down to synthesize glucose to supply organs, like the brain, that normally utilize glucose as a fuel. However, over a period of days, the body’s metabolism switches to the breakdown of ‘’fats’’, the storage form of fatty acids, which can be precursors for ketone bodies, an alternative fuel for the brain. This mechanism also works to the advantage of migratory birds, such as the ruby-throated hummingbird, which build up their fat stores before journeying long distances over water. The brain’s transition from glucose to ketone bodies occurs quite rapidly, so that hardly any protein in muscle is lost, enabling them to make their arduous, 2,400-kilometer flight.
The ruby-throated hummingbird
Excessive protein intake may be linked to some health problems:
- Liver dysfunction due to increased toxic residues. Because the body is unable to store excess protein, it is broken down and converted into sugars or fatty acids. The liver removes nitrogen from the amino acids, so that they can be burned as fuel, and the nitrogen is incorporated into urea, the substance that is excreted by the kidneys. These organs can normally cope with an extra workload but if kidney disease occurs, a decrease in protein will often be prescribed.
- Loss of bone density as calcium and glutamine are leached from bone and muscle tissue to balance increased acid intake from the diet. This effect is not present if intake of alkaline minerals is high. In such cases, protein intake helps to strengthen bones.
Studying proteins
The word protein was first mentioned in a letter sent by the Swedish chemist Jöns Jakob Berzelius to Gerhardus Johannes Mulder on July 10, 1838. He wrote:
The name protein that I propose for the organic oxide of fibrin and albumin, I wanted to derive from the Greek word πρωτειος, because it appears to be the primitive or principal substance of animal nutrition.
In twentieth-century study of proteins, one of the more striking discoveries was that the native and denatured states in many proteins were interconvertible (denatured refers to a protein that is not in its native state and is generally lacking a well-defined secondary structure). That is, by careful control of solution conditions to separate a denatured protein from the denaturing chemical, a denatured protein could be converted to its native form. The question of how proteins arrive at their native state is an important area of biochemistry, called the study of protein folding.
Through genetic engineering, researchers can alter the amino acid sequence and hence the structure, targeting, susceptibility to regulation, and other properties of a protein. The genetic sequences of different proteins may be spliced together to create chimeric proteins that possess properties of both. This form of tinkering represents one of the chief tools used by cell and molecular biologists to understand the workings of cells. Another area of protein research attempts to engineer proteins with entirely new properties or functions, a field known as protein engineering.
References
ISBN links support NWE through referral fees
- Atkins, P., and L. Jones. 2005. Chemical Principles, 3rd edition. New York: W. H. Freeman.
- Lewis, R. L. 2005. Do Proteins Teleport in an RNA World. New York: International Conference on the Unity of the Sciences.
- Stryer, L. 1995. Biochemistry, 4th edition. New York: W. H. Freeman.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article
in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Protein history
The history of this article since it was imported to New World Encyclopedia:
- History of «Protein»
Note: Some restrictions may apply to use of individual images which are separately licensed.
1
: any of various naturally occurring extremely complex substances that consist of amino-acid residues joined by peptide bonds, contain the elements carbon, hydrogen, nitrogen, oxygen, usually sulfur, and occasionally other elements (such as phosphorus or iron), and include many essential biological compounds (such as enzymes, hormones, or antibodies)
2
: the total nitrogenous material in plant or animal substances
Example Sentences
You need more protein in your diet.
These foods are an excellent source of protein.
These foods have all of the essential proteins.
Recent Examples on the Web
The goal is to create animal protein produced in electrified systems.
—
These abundant proteins randomly stick to cells throughout the body.
—
In the case of Fowler’s patient, the specific E. coli bacteria strain had a gene that makes a protein called NDM-1, which can break down even the strongest, last-resort antibiotics, called carbapenems.
—
Craig was charged with first-degree murder March 19 for spiking 43-year-old Angela Craig’s protein shakes with cyanide and arsenic, which left the mother of six brain-dead.
—
The company is exploring whether the model can predict levels of beta amyloid, the protein that builds up inside the brain in Alzheimer’s patients.
—
Unlike Botox, which uses human serum albumin, a protein derived from human blood, to act as the carrier, Daxxify utilizes a novel peptide technology to do the same.
—
Trace quantities of protein residue have long been detected in classic oil paintings, though they were often ascribed to contamination.
—
On page 7, the document describes a process for testing if the mRNA in the vaccine can lead to the production of a spiked protein, Russo said.
—
See More
These examples are programmatically compiled from various online sources to illustrate current usage of the word ‘protein.’ Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Etymology
borrowed from French protéine, from Late Greek prōteîos «of the first quality» (from Greek prôtos «first, foremost» + -eios, adjective suffix, originally from s-stems) + -ine -ine entry 1 — more at proto-
Note:
The term protein was introduced by the Dutch chemist Johannes Gerardus Mulder (1802-80), as French protéine in the article «Sur la composition de quelques substances animales» (Bulletin des sciences physiques et naturelles en Néerlande, vol. 1 [1838], pp. 104-19), and as Dutch protein in the article «Over Proteine en hare Verbindingen en Ontleidingsproducten» (Natuur- en scheikundig Archief, vol. 6 [1838], pp. 87-162). Though Mulder in the beginning of the papers expresses gratitude to Jöns Jakob berzelius for his support, he does not mention any connection between Berzelius and the novel word. In the twentieth century, however, it was discovered that Berzelius had suggested the word to Mulder in a letter written July 10, 1838: «Le nom protéine que je vous propose pour l’oxyde organique de la fibrine et de l’albumine, je voulais le dériver de πρωτειος, parce qu’il paraît être la substance primitive ou principale de la nutrition animale que les plantes préparent pour les herbivores et que ceux-ci fournissent ensuite aux carnassiers.» («The name protein, which I propose for the organic oxide of fibrin and albumin, I wish to derive from prōteios, because it appears to be the primitive or principal substance of animal nutrition, which plants prepare for herbivores, and which the latter then provide for carnivores.» — quoted in H.B. Vickery, «The origin of the word protein,» Yale Journal of Biology and Medicine, vol. 22, no. 5 [May, 1950], pp. 387-93.) In the French article, Mulder glosses the word prōteîos with Latin primarius «primary»: «The organic material, being a general principal of all the constituent parts of the animal body and being found, as we will see later, in the vegetable kingdom, could be named protein from prōteîos …» (La matière organique, étant un principe général de toutes les parties constituantes du corps animal, et se trouvant, comme nous verrons tantôt, dans le règne végétal, pourrait se nommer Protéine de πρωτεῖος primarius.») This appears to be Mulder’s own interpretation of the Greek word, as the leading Greek dictionary of the time, Franz Passow’s Handwörterbuch der griechischen Sprache (4. Ausgabe, 1831) defines it only as a masculine noun: «first rank, first place, primacy, priority» («erster Rang, erster Platz, Vorrang, Vorzug»). For details, see the article by H.B. Vickery cited above and Harold Hartley, «Origin of the Word ‘Protein’,» Nature, vol. 168, issue 4267 (August 11, 1951), p. 244.
First Known Use
1886, in the meaning defined at sense 1
Time Traveler
The first known use of protein was
in 1886
Dictionary Entries Near protein
Cite this Entry
“Protein.” Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/protein. Accessed 14 Apr. 2023.
Share
More from Merriam-Webster on protein
Last Updated:
31 Mar 2023
— Updated example sentences
Subscribe to America’s largest dictionary and get thousands more definitions and advanced search—ad free!
Merriam-Webster unabridged
Meaning Protein
What does Protein mean? Here you find 195 meanings of the word Protein. You can also add a definition of Protein yourself
1 |
0 One of the three nutrients used as energy sources (calories) by the body. Proteins are essential components of the muscle, skin, and bones. Proteins and carbohydrates each provide 4 calories of energy per gram, whereas fats provide 9 calories per gram.
|
2 |
0 ProteinSee: Acute-phase protein.
|
3 |
0 ProteinSee C-reactive protein.
|
4 |
0 ProteinSee: Proteolipid protein.
|
5 |
0 ProteinA molecule made up of amino acids. Proteins are needed for the body to function properly. They are the basis of body structures, such as skin and hair, and of other substances such as enzymes, cytokin [..]
|
6 |
0 ProteinOrganic substances primarily composed of carbon, hydrogen, nitrogen, and some other minor elements which are arranged in about 20 different compounds known as amino acids. The various amino acids foun [..]
|
7 |
0 Protein(pro-teen) [Gk. proteios, primary] A three-dimensional biological polymer constructed from a set of 20 different monomers called amino acids.
|
8 |
0 ProteinA complex polymer made by linking together amino acid molecules. Proteins sometimes contain non-amino acid components such as metal ions or porphyrin rings embedded within.
|
9 |
0 ProteinSubstance made up of amino acids that contains approximately 16% nitrogen (based on molecular weight).
|
10 |
0 Protein1 a substance in food such as meat, eggs, and milk that people need in order to grow and be healthySynonyms and related words Substances and chemicals in food and drink:additive, albumin, ascorbi [..]
|
11 |
0 ProteinProteins are large molecules that form the structural part of most organs and make up enzymes and hormones that regulate body functions.
|
12 |
0 ProteinA compound of organic acids; may contain carbon, hydrogen, nitrogen, or oxygen
|
13 |
0 Protein1844, from French protéine, coined 1838 by Dutch chemist Gerhard Johan Mulder (1802-1880), perhaps on suggestion of Berzelius, from Greek proteios «the first quality,» from protos «fir [..]
|
14 |
0 Proteinone of many complex compounds, made of chains of amino acids, that make up the majority of all cellular structures and are necessary for biological processes.
|
15 |
0 ProteinProteins are an important class of molecules found in all living cells. A protein is composed of one or more long chains of amino acids, the sequence of which corresponds to the DNA sequence of the gene that encodes it. Proteins play a variety of roles in the cell, including structural (cytoskeleton), mechanical (muscle), biochemical (enzymes), and [..]
|
16 |
0 ProteinA polymer of amino acids linked via peptide bonds and which may be composed of two or more polypeptide chains. 15 Animation
|
17 |
0 ProteinProteins are large organic compounds made of amino acids. They are essential to all living cells and organisms.
|
18 |
0 ProteinA polymer made up of amino acids and found in all living cells. Proteins that act as catalysts
|
19 |
0 Proteinextremely complex molecules of carbon, hydrogen, nitrogen, and other elements joined in chains of amino acids (peptides). Protein constitutes the bulk of living matter, gives it structure, and has something to do in almost every aspect of cell operation.
|
20 |
0 Proteinany of a large group of nitrogenous organic compounds that are essential constituents of living cells; consist of polymers of amino acids; essential in the diet of animals for growth and for repair of [..]
|
21 |
0 ProteinThe main building block of our cells. Each one has a specific function.
|
22 |
0 ProteinA connected series of amino acids that may have up to 20 different kinds of side chains; can exist in long fibrous or globular forms; component of macromolecules; forms enzymes and macromolecules acti [..]
|
23 |
0 ProteinA polypeptide or molecule made up of polypeptides. Examples: Albumin, hemoglobin, keratin.
|
24 |
0 Proteinprotein (pop)
|
25 |
0 ProteinProtein is one of the basic components of food and makes all life possible. Amino acids are the building blocks of proteins. All of the antibodies and enzymes, and many of the hormones in the body, ar [..]
|
26 |
0 ProteinProtein can be found in both animal and vegetable sources, and provides the body with energy while performing a large number of other functions.
|
27 |
0 ProteinLarge molecules composed of long chains of amino acids (see also amino acids). Essential for growth and repair, but also a source of energy with one gram of protein supplying four Calories when oxidised. Excess protein (amino acids) cannot be stored as such, therefore daily intake required.
|
28 |
0 ProteinA molecule made up of a sequence of amino acids (there are 23 different amino acids). Many of the important molecules in a living thing — for example, all enzymes — are proteins.
|
29 |
0 ProteinProteins are macromolecules made from twenty different types of amino acids. Proteins constitute the active component of cells . Proteins function as enzyme in metabolism, transporters and receptors in cell membranes, hormones, antibodies, and help read, translate, and replicate the genetic information.
|
30 |
0 ProteinWhile the genetic sequence provides the basic informational foundation of the cell, it is the network of protein- gene, protein- protein
|
31 |
0 Proteinlevel. Therefore, they provide an indirect and incomplete picture of cellular function
|
32 |
0 Proteina piece of cellular machinery. Each cell is made up of proteins which are created by ribosomes from the instruction set in an organism’s genome. Proteins are made up of a sequence of amino acids
|
33 |
0 Proteina basic constituent of all living cells. A biological macromolecule is composed of one or more amino acid chains linked by peptide bonds.
|
34 |
0 ProteinA long string of amino acids. The basic building material of organisms. Compare peptide.
|
35 |
0 ProteinA type of molecule composed of complex strings of amino acids (protein building blocks).
|
36 |
0 Proteinbiochemistry — important class of large biological molecules that usually contain a long and complexly folded structural unit (made from fatty acids or polysaccharides) with attached peptide side chains formed from amino acids
|
37 |
0 ProteinA type of polymer comprising a large number of monomers – a range of up to 20 amino acids – all connected by identical peptide links (covalent C-N bonds). Proteins are of three main types: globular proteins which are water-soluble (enzymes, antibodies, haemoglobin, insulin); membrane proteins which are particularly amphiphilic; fibrous proteins [..]
|
38 |
0 ProteinOne or several long biological polypeptide chains with specific amino-acid sequences. The polypeptide chains in proteins are usually folded into well defined 3-dimensional shapes, and the separate chains in a protein with more than one polypeptide are usually packed together with well-defined orientations. Proteins are classified broadly into globu [..]
|
39 |
0 ProteinAny of the ‘amino acids’ present in all living matter that are an essential food item.
|
40 |
0 ProteinBiological molecule consisting of linear chains of amino acids. Proteins are the principal products of genetic information and they do the bulk of work required for life. They perform a vast array of functions within living organisms, including providing cells with structure, mediating biochemical reactions, transmitting biological signals, and tra [..]
|
41 |
0 ProteinA molecule made up of a sequence of amino acids
|
42 |
0 Protein(French : protéine de structure) A protein that serves a structural role in the body such as collagen.
|
43 |
0 Proteina biomolecule that contains carbon, hydrogen, oxygen, and nitrogen; needed by the body for growth and repair
|
44 |
0 ProteinA molecule made up of amino acids that are needed for the body to function properly. Proteins are the basis of body structures such as skin and hair and of substances such as enzymes, cytokines, and antibodies.
|
45 |
0 ProteinA large molecule, made of amino acids, that is encoded by a gene or genes and that performs a specialised job in the cell.
|
46 |
0 ProteinProteins are large molecules required for the structure, function, and regulation of the body’s cells, tissues, and organs. Each protein has unique functions. Proteins are essential components of [..]
|
47 |
0 ProteinA molecule composed of one or more chains of amino acids in a specific order. Proteins are required for the structure, function, and regulation of the body’s cells, tissues, and organs, and each [..]
|
48 |
0 ProteinRequired for the structure, function and regulation of body cells, tissues and organs. A large molecule is made up of one or more chains of amino acids in a specific order. The order is determined by [..]
|
49 |
0 Proteinany of a group of large molecules that contain primarily carbon, hydrogen, oxygen, and nitrogen. Proteins are essential to the structure and function of all living cells. Examples of proteins in the b [..]
|
50 |
0 Protein(PRO-teen) 1. One of the three main nutrients in food. Foods that provide protein include meat, poultry, fish, cheese, milk, dairy products, eggs, and dried beans. 2. Proteins are also used in the body for cell structure, hormones such as insulin, and other functions.
|
51 |
0 ProteinComplex, nitrogen-containing substance that is found in food and is essential for the functioning of the human body. Protein molecules consist of long chains of building blocks called amino acids. Some of these amino acids can be manufactured in the human body. Others must be supplied by the diet. The body breaks down food proteins into their amino [..]
|
52 |
0 ProteinA large molecule composed of one or more chains of amino acids. An essential component of the human diet. In addition to providing amino acids needed for the assembly of proteins in our bodies, a gram [..]
|
53 |
0 ProteinUsed in professional kitchens as a general term to describe the meat, poultry, or fish in a presented dish.
|
54 |
0 Protein(n) any of a large group of nitrogenous organic compounds that are essential constituents of living cells; consist of polymers of amino acids; essential in the diet of animals for growth and for repa [..]
|
55 |
0 ProteinAn organic compound composed primarily of amino acids.
|
56 |
0 ProteinOne of the three major nutrients (along with carbohydrates and fats). It is used by the body for building and repairing tissues. Protein is derived primarily from animal sources but can be obtained fr [..]
|
57 |
0 ProteinMade up of amino acids, protein is essential for the growth and maintenance of body tissue, blood cells, hormones and enzymes. The best source of protein is fish, meat, poultry, milk products, and eggs.
|
58 |
0 ProteinThe loss of protein in the intestine.
|
59 |
0 ProteinA large complex molecule made up of one or more chains of amino acids. Proteins are needed by the body to grow and function properly.
|
60 |
0 ProteinDefinition: (PRO-teen) A molecule made up of amino acids that are needed for the body to function properly. Proteins are the basis of body structures such as skin and hair and of substances such as en [..]
|
61 |
0 ProteinProtein builds, maintains, and replaces the tissues in your body. (Not the tissues you blow your nose in! We mean the stuff your body’s made up of.) Your muscles, your organs, and your immune sys [..]
|
62 |
0 ProteinProtein is a nutrient important for the repair of tissues in the body. For adults, the recommended dietary intake for protein is about 0.75 g/kg body weight per day. Excess protein cannot be stored in [..]
|
63 |
0 Proteinin the diet it is required for tissue growth and repair. Composed of structural units called amino acids. Protein is not a significant energy source unless not enough calories and carbohydrate are consumed. One gram of protein equals four calories.
|
64 |
0 ProteinA linear polymer of amino acids linked together in a specific sequence and usually containing more than 50 residues. Proteins form the key structural elements in cells and participate in nearly all ce [..]
|
65 |
0 ProteinDefinition: Proteins are substances built up from amino acids. The construction of the individual proteins (the amino acid sequence) is stored in the DNA and is retrieved from there for the production. Proteins serve as a support structure and, in the form of hormones, as messenger substances. As enzymes they facilitate biochemical processes or fun [..]
|
66 |
0 ProteinA molecule composed of a long chain of amino acids. Proteins are the principal constituents of cellular material and serve as enzymes, hormones, structural elements, and antibodies. The molar mass is [..]
|
67 |
0 ProteinIllustrated Glossary of Organic Chemistry Protein: A peptide composed of many amino acids. Myoglobin, a protein containing 154 amino acid residues, used to bind oxygen in muscle cells. Structure from [..]
|
68 |
0 ProteinA molecule comprised of long chains of amino acid molecules. Proteins, which include enzymes, are polypeptides.
|
69 |
0 Proteina complex organic molecule composed of amino acids in a specific order. The order is determined by the sequence of nucleic acids in a gene coding for the protein. Proteins are required for the structu [..]
|
70 |
0 ProteinActivins are produced in the pituitary, Gonads, and other Tissues. By acting locally, they stimulate pituitary FSH Secretion and have diverse effects on Cell Differentiation and Embryonic Development. [..]
|
71 |
0 ProteinProteins coded by the retroviral gag Gene. The products are usually synthesized as protein precursors or Polyproteins, which are then cleaved by viral Proteases to yield the final products. Many of th [..]
|
72 |
0 ProteinPolyprotein products of a fused portion of retroviral mRNA containing the gag and pol Genes. The polyprotein is synthesized only five percent of the Time since pol is out of frame with gag, and is gen [..]
|
73 |
0 ProteinSulfur-Sulfur Bond Isomerases that catalyze the rearrangement of disulfide bonds within Proteins during folding. Specific protein disulfide-isomerase Isoenzymes also occur as subunits of Procollagen-P [..]
|
74 |
0 ProteinRegulatory Proteins that act as molecular switches. They control a wide range of Biological Processes including: receptor signaling, intracellular Signal Transduction pathways, and protein synthesis. [..]
|
75 |
0 ProteinProteins that contain an Iron-porphyrin, or Heme, prosthetic group resembling that of Hemoglobin. (From Lehninger, Principles of Biochemistry, 1982, p480)
|
76 |
0 ProteinA Transferase that catalyzes the addition of aliphatic, aromatic, or heterocyclic Free Radicals as well as Epoxides and arene Oxides to Glutathione. Addition takes place at the Sulfur. It also catalyz [..]
|
77 |
0 ProteinA Guanine Nucleotide Exchange Factor that is expressed primarily in neuronal Tissue and may be specific for the Ha-ras homolog of the ras Proteins.
|
78 |
0 ProteinGlycoprotein from Sendai, para-Influenza, Newcastle Disease, and other Viruses that participates in binding the Virus to Cell-surface receptors. The HN protein possesses both Hemagglutinin and Neurami [..]
|
79 |
0 ProteinHuD paraneoplastic Encephalomyelitis Antigen is an RNA-Binding Protein. It binds AU-rich sequences in the 3′ Untranslated Regions of mRNAs for Proto-Oncogene Proteins c-fos; Cyclin-Dependent Kina [..]
|
80 |
0 ProteinA multifunctional galactin initially discovered as a Macrophage Antigen that binds to Immunoglobulin E, and as 29-35-kDa lectin that binds Laminin. It is involved in a variety of biological events inc [..]
|
81 |
0 ProteinA product of the p16 Tumor Suppressor Gene (Genes, p16). It is also called INK4 or INK4A because it is the prototype member of the INK4 Cyclin-Dependent Kinase Inhibitors. This protein is produced fro [..]
|
82 |
0 ProteinA group of Enzymes that catalyzes the Hydrolysis of terminal, non-reducing beta-D-Galactose residues in beta-Galactosides. Deficiency of beta-Galactosidase A1 may cause Gangliosidosis, GM1.
|
83 |
0 ProteinA Ureahydrolase that catalyzes the Hydrolysis of Arginine or canavanine to yield L-Ornithine (Ornithine) and Urea. Deficiency of this enzyme causes Hyperargininemia. EC 3.5.3.1.
|
84 |
0 ProteinProteins which are found in Membranes including cellular and Intracellular Membranes. They consist of two types, peripheral and integral Proteins. They include most Membrane-associated Enzymes, antige [..]
|
85 |
0 ProteinThe major protein constituents of Milk are Caseins and whey Proteins such as LACTALBUMIN and Lactoglobulins. Immunoglobulins occur in high concentrations in Colostrum and in relatively lower concentra [..]
|
86 |
0 ProteinProteins encoded by the Mitochondrial Genome or Proteins encoded by the nuclear Genome that are imported to and resident in the Mitochondria.
|
87 |
0 ProteinA non-Heme Iron-Sulfur protein isolated from Clostridium pasteurianum and other Bacteria. It is a component of Nitrogenase, which is active in Nitrogen Fixation, and consists of two subunits with Mole [..]
|
88 |
0 ProteinA surface protein found on Plasmodium species which induces a T-Cell response. The Antigen is polymorphic, sharing Amino Acid Sequence Homology among Plasmodium falciparum; Plasmodium chabaudi; Plasmo [..]
|
89 |
0 ProteinThe protein constituents of Muscle, the major ones being Actins and Myosins. More than a dozen accessory Proteins exist including Troponin; Tropomyosin; and Dystrophin.
|
90 |
0 ProteinProteins produced from Genes that have acquired Mutations.
|
91 |
0 ProteinA myelin protein that is the major component of the organic solvent extractable Lipoprotein complexes of whole Brain. It has been the subject of much study because of its unusual physical properties. [..]
|
92 |
0 ProteinA myogenic regulatory factor that controls Myogenesis. Though it is not clear how its function differs from the other Myogenic Regulatory Factors, MyoD appears to be related to fusion and terminal dif [..]
|
93 |
0 ProteinATPases that are members of the AAA protein superFamily (ATPase Family Associated with various cellular Activities). The NSFs functions, acting in conjunction with Soluble NSF Attachment Proteins (i.e [..]
|
94 |
0 ProteinA heterotrimeric DNA-binding protein that binds to CCAAT motifs in the promoters of eukaryotic Genes. It is composed of three subunits: A, B and C.
|
95 |
0 ProteinSNARE binding Proteins that facilitate the ATP Hydrolysis-driven dissociation of the SNARE complex. They are required for the binding of N-Ethylmaleimide-Sensitive Protein (NSF) to the SNARE complex w [..]
|
96 |
0 ProteinA broad category of Nuclear Proteins that are components of or participate in the formation of the Nuclear Matrix.
|
97 |
0 ProteinAn organic cation transporter found in Kidney. It is localized to the basal lateral Membrane and is likely to be involved in the renal Secretion of organic Cations.
|
98 |
0 ProteinProteins, usually projecting from the Cilia of Olfactory Receptor Neurons, that specifically bind odorant molecules and trigger responses in the Neurons. The large number of different odorant receptor [..]
|
99 |
0 ProteinProteins coded by Oncogenes. They include Proteins resulting from the fusion of an Oncogene and another Gene (Oncogene Proteins, Fusion).
|
100 |
0 ProteinA protein that accounts for more than half of the Peripheral Nervous System myelin protein. The extraCellular domain of this protein is believed to engage in Adhesive interactions and thus hold the my [..]
|
101 |
0 ProteinA subtype of GPI-Anchored Folate Receptors that is expressed in Placenta and hematopoietic Cells.
|
102 |
0 ProteinRetroviral Proteins coded by the pol Gene. They are usually synthesized as a protein precursor (Polyproteins) and later cleaved into final products that include Reverse Transcriptase, endonuclease/Int [..]
|
103 |
0 ProteinA Glycoprotein migrating as alpha 1-globulin, Molecular Weight 70,000 to 120,000. The protein, which is present in increased amounts in the Plasma during Pregnancy, binds mainly Progesterone, with oth [..]
|
104 |
0 ProteinProteins found in Ribosomes. They are believed to have a catalytic function in reconstituting biologically active Ribosomal Subunits.
|
105 |
0 ProteinA subtype of Retinoic Acid Receptors that are specific for 9-cis-Retinoic Acid which function as nuclear Transcription Factors that regulate multiple signalling pathways.
|
106 |
0 ProteinA Membrane Protein homologous to the ERM (Ezrin-Radixin-Moesin) Family of Cytoskeleton-associated Proteins which regulate physical properties of Membranes. Alterations in Neurofibromin 2 are the cause [..]
|
107 |
0 ProteinA toxin produced by certain pathogenic strains of Escherichia coli such as Escherichia coli O157. It is closely related to Shiga Toxin produced by Shigella dysenteriae.
|
108 |
0 ProteinA subfamily of Q-SNARE Proteins which occupy the same position as syntaxin 1A in the SNARE complex and which also are most similar to syntaxin 1A in their Amino Acid Sequence. This subfamily is also k [..]
|
109 |
0 ProteinMicrotubule-Associated Proteins that are mainly expressed in Neurons. Tau Proteins constitute several Isoforms and play an important Role in the assembly of Tubulin monomers into Microtubules and in m [..]
|
110 |
0 ProteinProteins encoded by a Viral Genome that are produced in the organisms they infect, but not packaged into the Virus Particles. Some of these Proteins may play Roles within the infected Cell during Viru [..]
|
111 |
0 ProteinTrans-acting protein that combines with host factors to induce immediate early Gene transcription in Herpes Simplex Virus.
|
112 |
0 ProteinTransforming Proteins coded by rel Oncogenes. The v-rel protein competes with rel-related Proteins and probably transforms Cells by acting as a dominant negative version of c-rel. This results in the [..]
|
113 |
0 ProteinA Wnt protein subtype that plays a Role in Cell-Cell signaling during Embryonic Development and the Morphogenesis of the developing Neural Tube. Defects in Wnt3 protein are associated with autosomal r [..]
|
114 |
0 ProteinA Wnt protein subtype that plays a Role in Cell-Cell signaling during Embryonic Development and the Morphogenesis of the developing Neural Tube.
|
115 |
0 ProteinIsoforms encoded by the WT1 Wilms Tumor Suppressor Gene (Genes, Wilms Tumor) and produced by Alternative Splicings. They are Zinc Finger-containing Transcription Factors involved in both Transactivati [..]
|
116 |
0 ProteinTransforming protein coded by jun Oncogenes (Genes, jun). This is a gag-onc fusion protein of about 65 kDa derived from Avian Sarcoma Virus. v-jun lacks a negative regulatory domain that regulates tra [..]
|
117 |
0 ProteinProteins that are secreted into the Blood in increased or decreased quantities by Hepatocytes in response to Trauma, Inflammation, or Disease. These Proteins can serve as inhibitors or mediators of th [..]
|
118 |
0 ProteinConsists of a polypeptide chain and 4′-phosphopantetheine linked to a Serine residue by a phosphodiester bond. Acyl groups are bound as thiol esters to the pantothenyl group. Acyl carrier protein [..]
|
119 |
0 ProteinAn 86-amino acid polypeptide, found in central and peripheral Tissues, that displaces Diazepam from the Benzodiazepine recognition site on the gamma-Aminobutyric Acid Receptor (Receptors, GABA). It al [..]
|
120 |
0 ProteinCarrier Proteins produced in the Sertoli Cells of the Testis, secreted into the Seminiferous Tubules, and transported via the efferent ducts to the Epididymis. They participate in the transport of And [..]
|
121 |
0 ProteinA large and highly glycosylated protein constituent of Lipoprotein (a). It has very little affinity for Lipids but Forms disulfide-linkage to Apolipoprotein B-100. Apoprotein(a) has Serine Proteinase [..]
|
122 |
0 ProteinADP-Ribosylation Factor 1 is involved in regulating intracellular transport by modulating the interaction of Coat Proteins with Organelle Membranes in the early Secretory Pathway. It is a component of [..]
|
123 |
0 ProteinProteins found in any species of bacterium.
|
124 |
0 ProteinA 235-kDa cytoplasmic protein that is also found in Platelets. It has been localized to regions of Cell-substrate adhesion. It binds to Integrins; Vinculin; and Actins and appears to participate in ge [..]
|
125 |
0 ProteinA ubiquitous Membrane transport protein found in the Plasma Membrane of diverse Cell types and Tissues, and in nuclear, mitochondrial, and Golgi Membranes. It is the major integral transMembrane Prote [..]
|
126 |
0 ProteinA member of the Bcl-2 protein Family and homologous partner of C-BCL-2 PROTO-Oncogene Protein. It regulates the release of Cytochrome c and Apoptosis Inducing Factor from the Mitochondria. Several Iso [..]
|
127 |
0 ProteinAn abnormal protein with unusual thermosolubility characteristics that is found in the urine of Patients with Multiple Myeloma.
|
128 |
0 ProteinPeptides generated from Amyloid BETA-Peptides PRECURSOR. An Amyloid fibrillar form of these Peptides is the major component of Amyloid Plaques found in individuals with Alzheimer’s Disease and in [..]
|
129 |
0 ProteinA Family of F-box domain Proteins that contain sequences that are homologous to the beta subunit of Transducin (BETA-Transducin). They play an important Role in the Protein Degradation pathway by beco [..]
|
130 |
0 ProteinVitamin K-dependent Calcium-binding protein synthesized by Osteoblasts and found primarily in BONES. Serum osteocalcin measurements provide a noninvasive specific marker of bone Metabolism. The protei [..]
|
131 |
0 ProteinOne of the Virulence Factors produced by virulent Bordetella organisms. It is a bifunctional protein with both Adenylate Cyclase and hemolysin components.
|
132 |
0 ProteinA Protein-Serine-Threonine Kinase that is activated by Phosphorylation in response to Growth FACTORS or Insulin. It plays a major Role in Cell Metabolism, Growth, and Survival as a core component of S [..]
|
133 |
0 ProteinA Heat-stable, low-molecular-weight activator protein found mainly in the Brain and Heart. The binding of Calcium Ions to this protein allows this protein to bind to cyclic nucleotide Phosphodiesteras [..]
|
134 |
0 ProteinA transcriptional regulator in prokaryotes which, when activated by binding Cyclic AMP, acts at several promoters. Cyclic AMP Receptor protein was originally identified as a catabolite Gene activator [..]
|
135 |
0 ProteinA Chloride Channel that regulates Secretion in many exocrine Tissues. Abnormalities in the CFTR Gene have been shown to cause Cystic Fibrosis. (Hum Genet 1994;93(4):364-8)
|
136 |
0 ProteinSerum Glycoproteins participating in the host defense mechanism of Complement Activation that creates the Complement Membrane Attack Complex. Included are Glycoproteins in the various pathways of Comp [..]
|
137 |
0 ProteinA Serum protein that regulates the Classical Complement Activation Pathway. It binds as a cofactor to Complement Factor I which then hydrolyzes the Complement C4b in the Classical Pathway C3 Convertas [..]
|
138 |
0 ProteinA Plasma protein that circulates in increased amounts during Inflammation and after Tissue damage.
|
139 |
0 ProteinCellular DNA-Binding Proteins encoded by the rel Gene (Genes, rel). They are expressed predominately in hematopoietic Cells and may play a Role in Lymphocyte differentiation. Rel frequently combines w [..]
|
140 |
0 ProteinA Family of peptidyl-prolyl cis-trans Isomerases that bind to Cyclosporins and regulate the Immune System. EC 5.2.1.-
|
141 |
0 ProteinAn enzyme that catalyzes the desaturation (aromatization) of the ring A of C19 Androgens and converts them to C18 Estrogens. In this process, the 19-methyl is removed. This enzyme is Membrane-bound, l [..]
|
142 |
0 ProteinProteins obtained from Foods. They are the main source of the Essential Amino Acids.
|
143 |
0 ProteinProteins which are present in or isolated from vegetables or vegetable products used as Food. The concept is distinguished from Plant Proteins which refers to non-Dietary Proteins from Plants.
|
144 |
0 ProteinGlycosylated Proteins which are part of the salivary glue that Drosophila Larvae secrete as a means of fixing themselves to an external substrate for the duration of the pre-pupal and pupal period.
|
145 |
0 ProteinA Guanine Nucleotide Exchange Factor from Drosophila. Sevenless refers to genetic Mutations in Drosophila that cause loss of the R7 photoreceptor which is required to see UV Light.
|
146 |
0 ProteinA Family of RNA-Binding Proteins that are homologues of ELAV protein, Drosophila. They were initially identified in Humans as the targets of Autoantibodies in Patients with PARANEOPLASTIC Encephalomye [..]
|
147 |
0 ProteinA Family of Proteins that share the F-Box Motif and are involved in protein-protein interactions. They play an important Role in process of protein ubiquition by associating with a variety of substrat [..]
|
148 |
0 ProteinA Family of immunophilin Proteins that bind to the immunosuppressive Drugs Tacrolimus (also known as FK506) and Sirolimus. EC 5.2.1.-
|
149 |
0 ProteinA Serine Threonine Kinase that controls a wide range of Growth-related cellular processes. The protein is referred to as the target of Rapamycin due to the discovery that Tacrolimus (commonly known as [..]
|
150 |
0 ProteinA large molecule made up of a series of peptides—one or more long chains of amino acids. The specific sequence of the amino acids determines the protein’s structure and function. Proteins are essential to all living organisms. See Related Term(s): Amino Acids
|
151 |
0 ProteinA protein is a molecule made up of a sequence of amino acids. It is generally assumed that each protein is encoded by a single gene. Different proteins have different numbers of amino acids, but an av [..]
|
152 |
0 ProteinNutrients made up of carbon, hydrogen, oxygen and nitrogen, forming amino acids that help build the basic molecular structure of hair, skin, ligaments and muscles, as well as being essential to the pr [..]
|
153 |
0 ProteinOne of a group of high-molecular weight, nitrogen-containing organic compounds of complex shape and composition.
|
154 |
0 ProteinComplex molecules made of amino acids that include many substances (such as enzymes, hormones, and antibodies) necessary for the proper functioning of an organism.
|
155 |
0 ProteinGeneric term for the protein component of a dish—meat, fish, poultry, or even “meat substitute” like tofu or seitan. Does not refer to eggs.
|
156 |
0 ProteinAny of a class of nitrogenous organic compounds that consist of large molecules composed of one or more long chains of amino acids.
|
157 |
0 ProteinProtein is an essential nutrient. Proteins are composed of long chains of various kinds of amino acids. Animals meet their protein needs by breaking down plant and microbial protein (formed in the rumen) and reassembling them as animal proteins.
|
158 |
0 ProteinAny complex organic compound containing nitrogen.
|
159 |
0 ProteinA nutrient that is essential to the cat’s health. Cats cannot survive on vegetable proteins alone and must have meat in their diet. Cats require higher levels of protein than dogs and it is not s [..]
|
160 |
0 ProteinAny one of a class of naturally occurring compounds containing carbon, hydrogen, oxygen, nitrogen, often sulfur, phosphorus, occasionally iron and a few other elements. They are essentially very compl [..]
|
161 |
0 ProteinA molecule composed of one or more chains of amino acids in a specific order. Proteins are required for the structure, function and regulation of an organism’s cells and tissues, and each protein has a unique function.
|
162 |
0 Proteina type of organic compound that is one of the major components of cells and tissues.
|
163 |
0 Proteinare large organic compounds made of amino acids arranged in a linear chain and joined together by peptide bonds between the carboxyl and amino groups of adjacent amino acid residues.
|
164 |
0 ProteinA large molecule composed of one or more chains of up to several hundred amino acids in a specific order held together by peptide bonds. The order is determined by the base sequence of nucleotides in the gene that codes for the protein. Proteins are required for the structure, function and regulation of the body’s cells, tissues and organs, and e [..]
|
165 |
0 Proteinany molecule made up of amino acids; proteins form much of an organism’s structure and are responsible for many of its functions
|
166 |
0 ProteinA type of molecule composed of complex strings of amino acids (protein building blocks).
|
167 |
0 ProteinProteins do the work in cells. They can be part of structures (such as cell walls, organelles, etc), regulate reactions that take place in the cell or they can serve as enzymes, which speed-up reactio [..]
|
168 |
0 Proteina complex group of organic molecules that are the basic components of all living cells
|
169 |
0 ProteinA macromolecule made up on one or more polypeptide chains.
|
170 |
0 ProteinA molecule made up of amino acids that are needed for the body to function properly. Proteins are the basis of body structures such as skin and hair and of substances such as enzymes, cytokines and an [..]
|
171 |
0 ProteinComponents of cells and viruses that play structural and functional roles in cells.
|
172 |
0 ProteinThe building block for lots of parts of our bodies, for example muscles are made of proteins.
|
173 |
0 ProteinMatter found in living things with many roles, including helping to control how our cells work and fighting infections
|
174 |
0 ProteinA complex natural substance composed of amino acids useful in cheesemaking to form the web that holds the nutrients in the cheese and as a food source. (See Casein
|
175 |
0 ProteinA long-chain molecule consisting of amino acids. The function of a protein is determined by the sequence of amino acids. This sequence of amino acids is determined by the sequence of DNA bases found in the gene coding for that protein.
|
176 |
0 ProteinA molecule made up of amino acids that are needed for the body to function properly. Proteins are the basis of body structures such as skin and hair and of substances such as enzymes, cytokines, and antibodies. (Definition from: Physician Data Query via Unified Medical Language System
|
177 |
0 ProteinLarge biological molecules, or macromolecules, consisting of one or more long chains of amino acids. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by t [..]
|
178 |
0 ProteinA complex biological molecule composed of a chain of units called amino acids. Proteins have many different functions: structure(collagen); movement (actin and myosin); catalysis (enzymes); transport [..]
|
179 |
0 ProteinAn organic molecule that consists of amino acids joined together in a specific linear sequence. Proteins play a central role in biological processes and form the basis of living tissues. They have distinct and varied three-dimensional structures. Enzymes, antibodies and haemoglobin are examples of proteins.
|
180 |
0 ProteinComplex molecule containing carbon, hydrogen, oxygen and nitrogen; a major constituent of meat. Worms are approximately 60% protein.
|
181 |
0 ProteinA protein is a molecule which is a long chain (a polymer
|
182 |
0 ProteinAny of numerous naturally occurring extremely complex substances that consist of amino-acid residues joined by peptide bonds, contain the elements carbon, hydrogen, nitrogen, oxygen, usually sulfur, and occasionally other elements (as phosphorus or iron), and include many essential biological compounds (as enzymes, hormones, or immunoglobulins).
|
183 |
0 ProteinA linear biomacromolecule synthesized by ribosomes and consisting of a chain of amino acids in peptide linkage. Intracellular proteins have structural, regulatory, and catalytic functions; proteins secreted by cells have, in addition, intercellular signaling functions.
|
184 |
0 ProteinOrganic substances primarily composed of carbon, hydrogen, nitrogen, and some other minor elements which are arranged in about 20 different compounds known as amino acids. The various amino acids found in a protein are linked together by peptide bonds.
|
185 |
0 ProteinA naturally occurring complex organic substance present in relatively high amounts in meats, fish, eggs, cheese, legumes. Made of carbon, hydrogen, oxygen, and nitrogen, and sometimes sulfur and phosp [..]
|
186 |
0 ProteinMacromolecule (polymer) consisting of amino acids, which is an essential element for growth, repair, function and structure in all living cells. UV absorbs a maximum of 289 nm proteins.
|
187 |
0 ProteinA large molecule composed of one or more chains of amino acids in a specific order; the order is determined by the base sequence of nucleotides in the gene that codes for the protein. Proteins are req [..]
|
188 |
0 Proteina substance found in foods that the bodies of people and animals need to grow. pulp
|
189 |
0 ProteinA polymer of amino acids. See also the entry at NHGRI’s Talking Glossary of Genetic Terms.
|
190 |
0 ProteinComprised of amino acids, proteins are an essential nutrient group; in baking flour, “high protein” refers to the “strength” of the flour to produce gluten, comprised of two amino acids, glute [..]
|
191 |
0 Proteinis a major component of food. Good sources of protein include lean meat, fish, dairy products, eggs and legumes. Proteins are made up of smaller units called amino acids. There are 23 amino acids, e [..]
|
192 |
0 ProteinMacromolecules consisting of long sequences of amino acids. Protein is three-fourths of the dry weight of most cell matter and is involved in structures, hormones, enzymes, muscle contraction, immunol [..]
|
193 |
0 Proteinprotein
|
194 |
0 ProteinAn essential part of food which the body needs to repair itself and build muscle.
|
195 |
0 ProteinOne of the three types of nutrients that provides calories to the body. Protein is an essential nutrient that helps build many parts of the body, including muscle, bone, skin, and blood. Protein provides 4 calories per gram and is found in foods like meat, fish, poultry, eggs, dairy products, beans, nuts, and tofu.
|
Dictionary.university is a dictionary written by people like you and me.
Please help and add a word. All sort of words are welcome!
Add meaning