·
Next Topic
|
Posted: Thursday, December 29, 2016 11:15:02 AM |
Joined: 7/10/2016
Posts: 86
Neurons: 3,773
The glass (at least in one meaning) is a drinking vessel.
My question is: Is a glass always made of glass?
What is the name of a plastic drinking vessel? Is there such a thing as a plastic glass?
|
Posted: Thursday, December 29, 2016 11:54:35 AM |
Rank: Advanced Member
Joined: 10/7/2014
Posts: 230
Neurons: 841,732
Location: San Francisco, California, United States
As with almost everything in English, Yes and No
If going into a shop to buy wine glasses, when asking for them one would expect to be taken to glasses made from sand, silica or, recycled bottles or fine cut crystal
On the other hand, when asking a friend for a glass of juice, it could very well be given to you in a plastic vessel unless you specifically ask for a real glass.
therefore, depending on the nature of the request, the word «glass», can be used literally or metaphorically, objectively or subjectively.
Hope this helps
|
Posted: Thursday, December 29, 2016 12:05:28 PM |
Joined: 3/29/2012
Posts: 1,136
Neurons: 27,622
Location: Velké Meziříčí, Vysocina, Czech Republic
OnTheVerge wrote:
As with almost everything in English, Yes and No
If going into a shop to buy wine glasses, when asking for them one would expect to be taken to glasses made from sand, silica or, recycled bottles or fine cut crystal
I suppose you meant «…. one wouldn’t expect ….», or am I wrong?
|
Posted: Thursday, December 29, 2016 1:35:44 PM |
Rank: Advanced Member
Joined: 4/17/2009
Posts: 14,463
Neurons: 810,520
Location: Glasgow, Scotland, United Kingdom
I know OTV can speak for himself but I can’t resist saying that you are wrong, papo. One would expect to be taken to the Department that sells proper wine glasses — made of glass.
|
Posted: Thursday, December 29, 2016 2:11:52 PM |
Joined: 7/10/2016
Posts: 86
Neurons: 3,773
Thank you for the answers. I am reading The Wishsong of Shannara by Terry Brooks. It’s a fantasy tale but it is a postapoc fantasy because the scene is our Earth after a nuclear war changed the environment and the people. It is mostly a typical medieval fantasy but there are some things from our present for example flashlights or electric bulbs. These are rare though so our heroes use the classical fantasy equipment. And there was this scene where guests drank ale from glasses in an inn. And I couldn’t decide that these were glasses made of glass or really just clay/pewter mugs.
|
Posted: Thursday, December 29, 2016 4:02:56 PM |
Joined: 3/29/2012
Posts: 1,136
Neurons: 27,622
Location: Velké Meziříčí, Vysocina, Czech Republic
jacobusmaximus wrote:
I know OTV can speak for himself but I can’t resist saying that you are wrong, papo. One would expect to be taken to the Department that sells proper wine glasses — made of glass.
I see now, I didn’t realize at first that sand, silica,etc. are in fact raw materials used to make glass.
|
Posted: Thursday, December 29, 2016 5:00:47 PM |
Rank: Advanced Member
Joined: 4/20/2016
Posts: 2,703
Neurons: 189,102
Location: South Dublin, Ireland
Ages ago when I was young (not like now in my late 80s’) in Ireland, Dublin, I had been offered free of charge a shot in a plastic container filled with whiskey. It happened to me by Dawson Street. I think that most respected authorities on the question are ready to answer there inside this shop, located opposite Dublin Major’s manor. In strict confidence I may share the knowledge that, another shot free of charge may be possible, if not highly probable. Depending on the personal approach.
|
Posted: Friday, December 30, 2016 2:44:20 AM |
Rank: Advanced Member
Joined: 9/12/2011
Posts: 37,601
Neurons: 272,677
Location: Livingston, Scotland, United Kingdom
Hi Untergang.
If Terry Brooks said they drank from ‘glasses’, he probably meant
glass
. Anything else he would have called ‘mug’ or ‘tankard’.
If he said they had a glass of ale, it could have been in anything. A glass of ale is a measure — about ten fluid ounces, half a pint.
You can go into a pub and ask for a pint of ___ (whichever ale you fancy), or you can ask for a glass of ____.
Plastic things for drinking beer are normally called ‘plastic mugs’ or ‘plastic cups’ even if they are transparent (the ones you see provided near water-coolers and automatic coffee machines are sold as ‘disposable plastic cups’).
************
Have fun with Wishsong.
Have you read any of the books which deal with the actual atomic war and its aftermath, the sealing of Shannara and so on?
They’re quite fascinating.
|
Posted: Friday, December 30, 2016 3:18:08 AM |
Rank: Advanced Member
Joined: 9/21/2009
Posts: 47,927
Neurons: 676,083
Location: Helsinki, Southern Finland Province, Finland
I usually prefer my pint of good beer in a glassy mug, but in certain circumstances I can have it in a can 
|
Posted: Friday, December 30, 2016 3:23:01 AM |
Rank: Advanced Member
Joined: 3/30/2016
Posts: 3,882
Neurons: 25,759
Location: Luton, England, United Kingdom
In some venues here in the UK drinks can only be taken into them in ‘plastic glasses’ which are shatterproof, as real glasses can be used as weapons when broken, football grounds, pubs with a bad reputation etc. may have this rule enforced.
There are often known as ‘plastic glasses’ by many people, here I disagree with DragOnspeaker.
For example this website.
http://www.drinkstuff.com/products/plastic-glasses.asp
Although in most cases if something is just described as a glass or as a number of glasses in most cases it means vessels made of real glass.
The books before Shannara are quite good too, the Word and Void series that lead up to the war.
|
Posted: Friday, December 30, 2016 6:04:46 AM |
Joined: 7/10/2016
Posts: 86
Neurons: 3,773
Thank you, these answers were really helpful.
|
Posted: Friday, December 30, 2016 11:47:24 AM |
Rank: Advanced Member
Joined: 8/3/2016
Posts: 1,616
Neurons: 88,117
Location: Jandiāla Guru, Punjab, India
A ‘drinking vessel’ is a glass irrespective of the material it is made of.TFD
|
Posted: Monday, April 29, 2019 12:31:26 AM |
Rank: Newbie
Joined: 4/29/2019
Posts: 3
Neurons: 13
Location: Hook, England, United Kingdom
According to me, Glasses are made from the mixture of sand, lime and soda. When these ingredients are heated at an extreme temperate and then cooling down slightly at low temperature.
|
Posted: Monday, April 29, 2019 10:47:14 AM |
Joined: 7/21/2017
Posts: 195
Neurons: 1,152
The drinking vessel glass is not always made of glass. We have glasses made of gold, silver, bronze, brass, plastic and foam.
|
Posted: Tuesday, April 30, 2019 3:18:07 AM |
Rank: Advanced Member
Joined: 9/12/2011
Posts: 37,601
Neurons: 272,677
Location: Livingston, Scotland, United Kingdom
Hi!
Welcome to the Forum, Benjamin!
Is that Hook with Odiham Castle?
Islami — I think there is a difference in the way «glass» and «cup» are used between Britain and the Middle East (that’s where I guess you are from — somewhere between the Eastern Mediterranean and India).
You and Ashwin (from India) use «a glass» to mean any container for a drink, whereas Benjamin and I (from England) use «a glass» to mean one made from glass — I say «a plastic cup» or «a gold/silver/foam cup» for something like this. «A paper glass» doesn’t sound right
to me
, but (as Sarrriesfan says) «a plastic glass» is a known phrase here.
Another example of different usages — you DO hear «a plastic glass» over here, but not very often — «a plastic cup» is definitely preferred in Britain.
Here’s the graph of usage from Google.
Glass is a non-crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenching) of the molten form; some glasses such as volcanic glass are naturally occurring. The most familiar, and historically the oldest, types of manufactured glass are «silicate glasses» based on the chemical compound silica (silicon dioxide, or quartz), the primary constituent of sand. Soda–lime glass, containing around 70% silica, accounts for around 90% of manufactured glass. The term glass, in popular usage, is often used to refer only to this type of material, although silica-free glasses often have desirable properties for applications in modern communications technology. Some objects, such as drinking glasses and eyeglasses, are so commonly made of silicate-based glass that they are simply called by the name of the material.
Despite being brittle, buried silicate glass will survive for very long periods if not disturbed, and many examples of glass fragments exist from early glassmaking cultures. Archaeological evidence suggests glassmaking dates back to at least 3,600 BC in Mesopotamia, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience. Due to its ease of formability into any shape, glass has been traditionally used for vessels, such as bowls, vases, bottles, jars and drinking glasses. In its most solid forms, it has also been used for paperweights and marbles. Glass can be coloured by adding metal salts or painted and printed with vitreous enamels, leading to its use in stained glass windows and other glass art objects. The refractive, reflective and transmission properties of glass make glass suitable for manufacturing optical lenses, prisms, and optoelectronics materials. Extruded glass fibres have application as optical fibres in communications networks, thermal insulating material when matted as glass wool so as to trap air, or in glass-fibre reinforced plastic (fibreglass).
Microscopic structure
The amorphous structure of glassy silica (SiO2) in two dimensions. No long-range order is present, although there is local ordering with respect to the tetrahedral arrangement of oxygen (O) atoms around the silicon (Si) atoms.
Microscopically, a single crystal has atoms in a near-perfect periodic arrangement; a polycrystal is composed of many microscopic crystals; and an amorphous solid such as glass has no periodic arrangement even microscopically.
The standard definition of a glass (or vitreous solid) is a solid formed by rapid melt quenching.[1][2][3][4] However, the term «glass» is often defined in a broader sense, to describe any non-crystalline (amorphous) solid that exhibits a glass transition when heated towards the liquid state.[4][5]
Glass is an amorphous solid. Although the atomic-scale structure of glass shares characteristics of the structure of a supercooled liquid, glass exhibits all the mechanical properties of a solid.[6][7][8] As in other amorphous solids, the atomic structure of a glass lacks the long-range periodicity observed in crystalline solids. Due to chemical bonding constraints, glasses do possess a high degree of short-range order with respect to local atomic polyhedra.[9] The notion that glass flows to an appreciable extent over extended periods of time is not supported by empirical research or theoretical analysis (see viscosity in solids). Though a material viscosity on the order of 1017–1018 Pa s can be measured in glass, such a high value reinforces the fact that glass would not change shape appreciably over even large periods of time.[5][10]
Formation from a supercooled liquid
Unsolved problem in physics :
What is the nature of the transition between a fluid or regular solid and a glassy phase?
«The deepest and most interesting unsolved problem in solid state theory is probably the theory of the nature of glass and the glass transition.» —P.W. Anderson[11]
For melt quenching, if the cooling is sufficiently rapid (relative to the characteristic crystallization time) then crystallization is prevented and instead the disordered atomic configuration of the supercooled liquid is frozen into the solid state at Tg. The tendency for a material to form a glass while quenched is called glass-forming ability. This ability can be predicted by the rigidity theory.[12] Generally, a glass exists in a structurally metastable state with respect to its crystalline form, although in certain circumstances, for example in atactic polymers, there is no crystalline analogue of the amorphous phase.[13]
Glass is sometimes considered to be a liquid due to its lack of a first-order phase transition[7][14]
where certain thermodynamic variables such as volume, entropy and enthalpy are discontinuous through the glass transition range. The glass transition may be described as analogous to a second-order phase transition where the intensive thermodynamic variables such as the thermal expansivity and heat capacity are discontinuous.[2] However, the equilibrium theory of phase transformations does not hold for glass, and hence the glass transition cannot be classed as one of the classical equilibrium phase transformations in solids.[4][5]
Occurrence in nature
Glass can form naturally from volcanic magma. Obsidian is a common volcanic glass with high silica (SiO2) content formed when felsic lava extruded from a volcano cools rapidly.[15] Impactite is a form of glass formed by the impact of a meteorite, where Moldavite (found in central and eastern Europe), and Libyan desert glass (found in areas in the eastern Sahara, the deserts of eastern Libya and western Egypt) are notable examples.[16] Vitrification of quartz can also occur when lightning strikes sand, forming hollow, branching rootlike structures called fulgurites.[17] Trinitite is a glassy residue formed from the desert floor sand at the Trinity nuclear bomb test site.[18] Edeowie glass, found in South Australia, is proposed to originate from Pleistocene grassland fires, lightning strikes, or hypervelocity impact by one or several asteroids or comets.[19]
History
Naturally occurring obsidian glass was used by Stone Age societies as it fractures along very sharp edges, making it ideal for cutting tools and weapons.[20][21] Glassmaking dates back at least 6000 years, long before humans had discovered how to smelt iron.[20] Archaeological evidence suggests that the first true synthetic glass was made in Lebanon and the coastal north Syria, Mesopotamia or ancient Egypt.[22][23] The earliest known glass objects, of the mid-third millennium BC, were beads, perhaps initially created as accidental by-products of metalworking (slags) or during the production of faience, a pre-glass vitreous material made by a process similar to glazing.[24]
Early glass was rarely transparent and often contained impurities and imperfections,[20] and is technically faience rather than true glass, which did not appear until the 15th century BC.[25] However, red-orange glass beads excavated from the Indus Valley Civilization dated before 1700 BC (possibly as early as 1900 BC) predate sustained glass production, which appeared around 1600 BC in Mesopotamia and 1500 BC in Egypt.[26][27] During the Late Bronze Age there was a rapid growth in glassmaking technology in Egypt and Western Asia.[22] Archaeological finds from this period include coloured glass ingots, vessels, and beads.[22][28] Much early glass production relied on grinding techniques borrowed from stoneworking, such as grinding and carving glass in a cold state.[29]
The term glass developed in the late Roman Empire. It was in the Roman glassmaking centre at Trier (located in current-day Germany) that the late-Latin term glesum originated, probably from a Germanic word for a transparent, lustrous substance.[30] Glass objects have been recovered across the Roman Empire[31] in domestic, funerary,[32] and industrial contexts,[33] as well as trade items in marketplaces in distant provinces.[34][35] Examples of Roman glass have been found outside of the former Roman Empire in China,[36] the Baltics, the Middle East, and India.[37] The Romans perfected cameo glass, produced by etching and carving through fused layers of different colours to produce a design in relief on the glass object.[38]
Windows in the choir of the Basilica of Saint-Denis, one of the earliest uses of extensive areas of glass (early 13th-century architecture with restored glass of the 19th century)
In post-classical West Africa, Benin was a manufacturer of glass and glass beads.[39]
Glass was used extensively in Europe during the Middle Ages. Anglo-Saxon glass has been found across England during archaeological excavations of both settlement and cemetery sites.[40] From the 10th century onwards, glass was employed in stained glass windows of churches and cathedrals, with famous examples at Chartres Cathedral and the Basilica of Saint-Denis. By the 14th century, architects were designing buildings with walls of stained glass such as Sainte-Chapelle, Paris, (1203–1248) and the East end of Gloucester Cathedral. With the change in architectural style during the Renaissance period in Europe, the use of large stained glass windows became much less prevalent,[41] although stained glass had a major revival with Gothic Revival architecture in the 19th century.[42]
During the 13th century, the island of Murano, Venice, became a centre for glass making, building on medieval techniques to produce colourful ornamental pieces in large quantities.[38] Murano glass makers developed the exceptionally clear colourless glass cristallo, so called for its resemblance to natural crystal, which was extensively used for windows, mirrors, ships’ lanterns, and lenses.[20] In the 13th, 14th, and 15th centuries, enamelling and gilding on glass vessels was perfected in Egypt and Syria.[43] Towards the end of the 17th century, Bohemia became an important region for glass production, remaining so until the start of the 20th century. By the 17th century, glass in the Venetian tradition was also being produced in England. In about 1675, George Ravenscroft invented lead crystal glass, with cut glass becoming fashionable in the 18th century.[38] Ornamental glass objects became an important art medium during the Art Nouveau period in the late 19th century.[38]
Throughout the 20th century, new mass production techniques led to widespread availability of glass in much larger amounts, making it practical as a building material and enabling new applications of glass.[44] In the 1920s a mould-etch process was developed, in which art was etched directly into the mould, so that each cast piece emerged from the mould with the image already on the surface of the glass. This reduced manufacturing costs and, combined with a wider use of coloured glass, led to cheap glassware in the 1930s, which later became known as Depression glass.[45] In the 1950s, Pilkington Bros., England, developed the float glass process, producing high-quality distortion-free flat sheets of glass by floating on molten tin.[20] Modern multi-story buildings are frequently constructed with curtain walls made almost entirely of glass.[46] Laminated glass has been widely applied to vehicles for windscreens.[47] Optical glass for spectacles has been used since the Middle Ages.[48] The production of lenses has become increasingly proficient, aiding astronomers[49] as well as having other application in medicine and science.[50] Glass is also employed as the aperture cover in many solar energy collectors.[51]
In the 21st century, glass manufacturers have developed different brands of chemically strengthened glass for widespread application in touchscreens for smartphones, tablet computers, and many other types of information appliances. These include Gorilla Glass, developed and manufactured by Corning, AGC Inc.’s Dragontrail and Schott AG’s Xensation.[52][53][54]
Physical properties
Optical
Glass is in widespread use in optical systems due to its ability to refract, reflect, and transmit light following geometrical optics. The most common and oldest applications of glass in optics are as lenses, windows, mirrors, and prisms.[55] The key optical properties refractive index, dispersion, and transmission, of glass are strongly dependent on chemical composition and, to a lesser degree, its thermal history.[55] Optical glass typically has a refractive index of 1.4 to 2.4, and an Abbe number (which characterises dispersion) of 15 to 100.[55] Refractive index may be modified by high-density (refractive index increases) or low-density (refractive index decreases) additives.[56]
Glass transparency results from the absence of grain boundaries which diffusely scatter light in polycrystalline materials.[57] Semi-opacity due to crystallization may be induced in many glasses by maintaining them for a long period at a temperature just insufficient to cause fusion. In this way, the crystalline, devitrified material, known as Réaumur’s glass porcelain is produced.[43][58] Although generally transparent to visible light, glasses may be opaque to other wavelengths of light. While silicate glasses are generally opaque to infrared wavelengths with a transmission cut-off at 4 μm, heavy-metal fluoride and chalcogenide glasses are transparent to infrared wavelengths of 7 to 18 μm.[59] The addition of metallic oxides results in different coloured glasses as the metallic ions will absorb wavelengths of light corresponding to specific colours.[59]
Other
In the manufacturing process, glasses can be poured, formed, extruded and moulded into forms ranging from flat sheets to highly intricate shapes.[60] The finished product is brittle and will fracture, unless laminated or tempered to enhance durability.[61][62] Glass is typically inert, resistant to chemical attack, and can mostly withstand the action of water, making it an ideal material for the manufacture of containers for foodstuffs and most chemicals.[20][63][64] Nevertheless, although usually highly resistant to chemical attack, glass will corrode or dissolve under some conditions.[63][65] The materials that make up a particular glass composition have an effect on how quickly the glass corrodes. Glasses containing a high proportion of alkali or alkaline earth elements are more susceptible to corrosion than other glass compositions.[66][67]
The density of glass varies with chemical composition with values ranging from 2.2 grams per cubic centimetre (2,200 kg/m3) for fused silica to 7.2 grams per cubic centimetre (7,200 kg/m3) for dense flint glass.[68] Glass is stronger than most metals, with a theoretical tensile strength for pure, flawless glass estimated at 14 to 35 gigapascals (2,000,000 to 5,100,000 psi) due to its ability to undergo reversible compression without fracture. However, the presence of scratches, bubbles, and other microscopic flaws lead to a typical range of 14 to 175 megapascals (2,000 to 25,400 psi) in most commercial glasses.[59] Several processes such as toughening can increase the strength of glass.[69] Carefully drawn flawless glass fibres can be produced with strength of up to 11.5 gigapascals (1,670,000 psi).[59]
Reputed flow
The observation that old windows are sometimes found to be thicker at the bottom than at the top is often offered as supporting evidence for the view that glass flows over a timescale of centuries, the assumption being that the glass has exhibited the liquid property of flowing from one shape to another.[70] This assumption is incorrect, as once solidified, glass stops flowing. The sags and ripples observed in old glass were already there the day it was made; manufacturing processes used in the past produced sheets with imperfect surfaces and non-uniform thickness (the near-perfect float glass used today only became widespread in the 1960s). [7]
A 2017 study computed the rate of flow of the medieval glass used in Westminster Abbey from the year 1268. The study found that the room temperature viscosity of this glass was roughly 1024 Pa·s which is about 1016 times less viscous than a previous estimate made in 1998, which focused on soda-lime silicate glass. Even with this lower viscosity, the study authors calculated that the maximum flow rate of medieval glass is 1nm per billion years, making it impossible to observe in a human timescale.[71][72]
Types
Silicate
Quartz sand (silica) is the main raw material in commercial glass production
Silicon dioxide (SiO2) is a common fundamental constituent of glass. Fused quartz is a glass made from chemically pure silica.[67] It has very low thermal expansion and excellent resistance to thermal shock, being able to survive immersion in water while red hot, resists high temperatures (1000–1500 °C) and chemical weathering, and is very hard. It is also transparent to a wider spectral range than ordinary glass, extending from the visible further into both the UV and IR ranges, and is sometimes used where transparency to these wavelengths is necessary. Fused quartz is used for high-temperature applications such as furnace tubes, lighting tubes, melting crucibles, etc.[73] However, its high melting temperature (1723 °C) and viscosity make it difficult to work with. Therefore, normally, other substances (fluxes) are added to lower the melting temperature and simplify glass processing.[74]
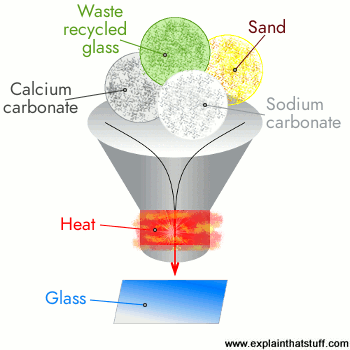
Soda–lime
Sodium carbonate (Na2CO3, «soda») is a common additive and acts to lower the glass-transition temperature. However, sodium silicate is water-soluble, so lime (CaO, calcium oxide, generally obtained from limestone), along with magnesium oxide (MgO), and aluminium oxide (Al2O3), are commonly added to improve chemical durability. Soda–lime glasses (Na2O) + lime (CaO) + magnesia (MgO) + alumina (Al2O3) account for over 75% of manufactured glass, containing about 70 to 74% silica by weight.[67][75] Soda–lime–silicate glass is transparent, easily formed, and most suitable for window glass and tableware.[76] However, it has a high thermal expansion and poor resistance to heat.[76] Soda–lime glass is typically used for windows, bottles, light bulbs, and jars.[74]
Borosilicate
Borosilicate glasses (e.g. Pyrex, Duran) typically contain 5–13% boron trioxide (B2O3).[74] Borosilicate glasses have fairly low coefficients of thermal expansion (7740 Pyrex CTE is 3.25×10−6/°C[77] as compared to about 9×10−6/°C for a typical soda–lime glass[78]). They are, therefore, less subject to stress caused by thermal expansion and thus less vulnerable to cracking from thermal shock. They are commonly used for e.g. labware, household cookware, and sealed beam car head lamps.[74]
Lead
The addition of lead(II) oxide into silicate glass lowers melting point and viscosity of the melt.[79] The high density of lead glass (silica + lead oxide (PbO) + potassium oxide (K2O) + soda (Na2O) + zinc oxide (ZnO) + alumina) results in a high electron density, and hence high refractive index, making the look of glassware more brilliant and causing noticeably more specular reflection and increased optical dispersion.[67][80] Lead glass has a high elasticity, making the glassware more workable and giving rise to a clear «ring» sound when struck. However, lead glass cannot withstand high temperatures well.[73] Lead oxide also facilitates solubility of other metal oxides and is used in coloured glass. The viscosity decrease of lead glass melt is very significant (roughly 100 times in comparison with soda glass); this allows easier removal of bubbles and working at lower temperatures, hence its frequent use as an additive in vitreous enamels and glass solders. The high ionic radius of the Pb2+ ion renders it highly immobile and hinders the movement of other ions; lead glasses therefore have high electrical resistance, about two orders of magnitude higher than soda–lime glass (108.5 vs 106.5 Ω⋅cm, DC at 250 °C).[81]
Aluminosilicate
Aluminosilicate glass typically contains 5–10% alumina (Al2O3). Aluminosilicate glass tends to be more difficult to melt and shape compared to borosilicate compositions, but has excellent thermal resistance and durability.[74] Aluminosilicate glass is extensively used for fiberglass,[82] used for making glass-reinforced plastics (boats, fishing rods, etc.), top-of-stove cookware, and halogen bulb glass.[73][74]
Other oxide additives
The addition of barium also increases the refractive index. Thorium oxide gives glass a high refractive index and low dispersion and was formerly used in producing high-quality lenses, but due to its radioactivity has been replaced by lanthanum oxide in modern eyeglasses.[83] Iron can be incorporated into glass to absorb infrared radiation, for example in heat-absorbing filters for movie projectors, while cerium(IV) oxide can be used for glass that absorbs ultraviolet wavelengths.[84] Fluorine lowers the dielectric constant of glass. Fluorine is highly electronegative and lowers the polarizability of the material. Fluoride silicate glasses are used in manufacture of integrated circuits as an insulator.[85]
Glass-ceramics
Glass-ceramic materials contain both non-crystalline glass and crystalline ceramic phases. They are formed by controlled nucleation and partial crystallisation of a base glass by heat treatment.[86] Crystalline grains are often embedded within a non-crystalline intergranular phase of grain boundaries. Glass-ceramics exhibit advantageous thermal, chemical, biological, and dielectric properties as compared to metals or organic polymers.[86]
The most commercially important property of glass-ceramics is their imperviousness to thermal shock. Thus, glass-ceramics have become extremely useful for countertop cooking and industrial processes. The negative thermal expansion coefficient (CTE) of the crystalline ceramic phase can be balanced with the positive CTE of the glassy phase. At a certain point (~70% crystalline) the glass-ceramic has a net CTE near zero. This type of glass-ceramic exhibits excellent mechanical properties and can sustain repeated and quick temperature changes up to 1000 °C.[87][86]
Fibreglass
Fibreglass (also called glass fibre reinforced plastic, GRP) is a composite material made by reinforcing a plastic resin with glass fibres. It is made by melting glass and stretching the glass into fibres. These fibres are woven together into a cloth and left to set in a plastic resin.[88][89][90]
Fibreglass has the properties of being lightweight and corrosion resistant, and is a good insulator enabling its use as building insulation material and for electronic housing for consumer products. Fibreglass was originally used in the United Kingdom and United States during World War II to manufacture radomes. Uses of fibreglass include building and construction materials, boat hulls, car body parts, and aerospace composite materials.[91][88][90]
Glass-fibre wool is an excellent thermal and sound insulation material, commonly used in buildings (e.g. attic and cavity wall insulation), and plumbing (e.g. pipe insulation), and soundproofing.[91] It is produced by forcing molten glass through a fine mesh by centripetal force, and breaking the extruded glass fibres into short lengths using a stream of high-velocity air. The fibres are bonded with an adhesive spray and the resulting wool mat is cut and packed in rolls or panels.[59]
Non-silicate
Besides common silica-based glasses many other inorganic and organic materials may also form glasses, including metals, aluminates, phosphates, borates, chalcogenides, fluorides, germanates (glasses based on GeO2), tellurites (glasses based on TeO2), antimonates (glasses based on Sb2O3), arsenates (glasses based on As2O3), titanates (glasses based on TiO2), tantalates (glasses based on Ta2O5), nitrates, carbonates, plastics, acrylic, and many other substances.[5] Some of these glasses (e.g. Germanium dioxide (GeO2, Germania), in many respects a structural analogue of silica, fluoride, aluminate, phosphate, borate, and chalcogenide glasses) have physico-chemical properties useful for their application in fibre-optic waveguides in communication networks and other specialised technological applications.[93][94]
Silica-free glasses may often have poor glass forming tendencies. Novel techniques, including containerless processing by aerodynamic levitation (cooling the melt whilst it floats on a gas stream) or splat quenching (pressing the melt between two metal anvils or rollers), may be used to increase cooling rate, or to reduce crystal nucleation triggers.[95][96][97]
Amorphous metals
Samples of amorphous metal, with millimeter scale
In the past, small batches of amorphous metals with high surface area configurations (ribbons, wires, films, etc.) have been produced through the implementation of extremely rapid rates of cooling. Amorphous metal wires have been produced by sputtering molten metal onto a spinning metal disk.[98][99]
A number of alloys have been produced in layers with thickness exceeding 1 millimeter. These are known as bulk metallic glasses (BMG). Liquidmetal Technologies sell a number of zirconium-based BMGs.
Batches of amorphous steel have also been produced that demonstrate mechanical properties far exceeding those found in conventional steel alloys.[100]
Experimental evidence indicates that the system Al-Fe-Si may undergo a first-order transition to an amorphous form (dubbed «q-glass») on rapid cooling from the melt. Transmission electron microscopy (TEM) images indicate that q-glass nucleates from the melt as discrete particles with a uniform spherical growth in all directions. While x-ray diffraction reveals the isotropic nature of q-glass, a nucleation barrier exists implying an interfacial discontinuity (or internal surface) between the glass and melt phases.[101][102]
Polymers
Important polymer glasses include amorphous and glassy pharmaceutical compounds. These are useful because the solubility of the compound is greatly increased when it is amorphous compared to the same crystalline composition. Many emerging pharmaceuticals are practically insoluble in their crystalline forms.[103] Many polymer thermoplastics familiar from everyday use are glasses. For many applications, like glass bottles or eyewear, polymer glasses (acrylic glass, polycarbonate or polyethylene terephthalate) are a lighter alternative to traditional glass.[104]
Molecular liquids and molten salts
Molecular liquids, electrolytes, molten salts, and aqueous solutions are mixtures of different molecules or ions that do not form a covalent network but interact only through weak van der Waals forces or through transient hydrogen bonds. In a mixture of three or more ionic species of dissimilar size and shape, crystallization can be so difficult that the liquid can easily be supercooled into a glass.[105][106] Examples include LiCl:RH2O (a solution of lithium chloride salt and water molecules) in the composition range 4<R<8.[107] sugar glass,[108] or Ca0.4K0.6(NO3)1.4.[109] Glass electrolytes in the form of Ba-doped Li-glass and Ba-doped Na-glass have been proposed as solutions to problems identified with organic liquid electrolytes used in modern lithium-ion battery cells.[110]
Production
Robotised float glass unloading
Following the glass batch preparation and mixing, the raw materials are transported to the furnace. Soda–lime glass for mass production is melted in glass melting furnaces. Smaller scale furnaces for specialty glasses include electric melters, pot furnaces, and day tanks.[75]
After melting, homogenization and refining (removal of bubbles), the glass is formed. Flat glass for windows and similar applications is formed by the float glass process, developed between 1953 and 1957 by Sir Alastair Pilkington and Kenneth Bickerstaff of the UK’s Pilkington Brothers, who created a continuous ribbon of glass using a molten tin bath on which the molten glass flows unhindered under the influence of gravity. The top surface of the glass is subjected to nitrogen under pressure to obtain a polished finish.[111] Container glass for common bottles and jars is formed by blowing and pressing methods.[112] This glass is often slightly modified chemically (with more alumina and calcium oxide) for greater water resistance.[113]
Once the desired form is obtained, glass is usually annealed for the removal of stresses and to increase the glass’s hardness and durability.[114] Surface treatments, coatings or lamination may follow to improve the chemical durability (glass container coatings, glass container internal treatment), strength (toughened glass, bulletproof glass, windshields[115]), or optical properties (insulated glazing, anti-reflective coating).[116]
New chemical glass compositions or new treatment techniques can be initially investigated in small-scale laboratory experiments. The raw materials for laboratory-scale glass melts are often different from those used in mass production because the cost factor has a low priority. In the laboratory mostly pure chemicals are used. Care must be taken that the raw materials have not reacted with moisture or other chemicals in the environment (such as alkali or alkaline earth metal oxides and hydroxides, or boron oxide), or that the impurities are quantified (loss on ignition).[117] Evaporation losses during glass melting should be considered during the selection of the raw materials, e.g., sodium selenite may be preferred over easily evaporating selenium dioxide (SeO2). Also, more readily reacting raw materials may be preferred over relatively inert ones, such as aluminum hydroxide (Al(OH)3) over alumina (Al2O3). Usually, the melts are carried out in platinum crucibles to reduce contamination from the crucible material. Glass homogeneity is achieved by homogenizing the raw materials mixture (glass batch), by stirring the melt, and by crushing and re-melting the first melt. The obtained glass is usually annealed to prevent breakage during processing.[117][118]
Colour
Colour in glass may be obtained by addition of homogenously distributed electrically charged ions (or colour centres). While ordinary soda–lime glass appears colourless in thin section, iron(II) oxide (FeO) impurities produce a green tint in thick sections.[119] Manganese dioxide (MnO2), which gives glass a purple colour, may be added to remove the green tint given by FeO.[120] FeO and chromium(III) oxide (Cr2O3) additives are used in the production of green bottles.[119] Iron (III) oxide, on the other-hand, produces yellow or yellow-brown glass.[121] Low concentrations (0.025 to 0.1%) of cobalt oxide (CoO) produces rich, deep blue cobalt glass.[122] Chromium is a very powerful colourising agent, yielding dark green.[123]
Sulphur combined with carbon and iron salts produces amber glass ranging from yellowish to almost black.[124] A glass melt can also acquire an amber colour from a reducing combustion atmosphere.[125] Cadmium sulfide produces imperial red, and combined with selenium can produce shades of yellow, orange, and red.[119][121] The additive Copper(II) oxide (CuO) produces a turquoise colour in glass, in contrast to Copper(I) oxide (Cu2O) which gives a dull brown-red colour.[126]
-
-
Red glass bottle with yellow glass overlay
-
Amber-coloured glass
-
Four-colour Roman glass bowl, manufactured circa 1st century B.C.
Uses
Architecture and windows
Soda–lime sheet glass is typically used as transparent glazing material, typically as windows in external walls of buildings. Float or rolled sheet glass products is cut to size either by scoring and snapping the material, laser cutting, water jets, or diamond bladed saw. The glass may be thermally or chemically tempered (strengthened) for safety and bent or curved during heating. Surface coatings may be added for specific functions such as scratch resistance, blocking specific wavelengths of light (e.g. infrared or ultraviolet), dirt-repellence (e.g. self-cleaning glass), or switchable electrochromic coatings.[127]
Structural glazing systems represent one of the most significant architectural innovations of modern times, where glass buildings now often dominate skylines of many modern cities.[128] These systems use stainless steel fittings countersunk into recesses in the corners of the glass panels allowing strengthened panes to appear unsupported creating a flush exterior.[128] Structural glazing systems have their roots in iron and glass conservatories of the nineteenth century[129]
Tableware
Glass is an essential component of tableware and is typically used for water, beer and wine drinking glasses.[50] Wine glasses are typically stemware, i.e. goblets formed from a bowl, stem, and foot. Crystal or Lead crystal glass may be cut and polished to produce decorative drinking glasses with gleaming facets.[130][131] Other uses of glass in tableware include decanters, jugs, plates, and bowls.[50]
-
Wine glasses and other glass tableware
-
Dimpled glass beer pint jug
-
-
Packaging
The inert and impermeable nature of glass makes it a stable and widely used material for food and drink packaging as glass bottles and jars. Most container glass is soda–lime glass, produced by blowing and pressing techniques. Container glass has a lower magnesium oxide and sodium oxide content than flat glass, and a higher silica, calcium oxide, and aluminum oxide content.[132] Its higher content of water-insoluble oxides imparts slightly higher chemical durability against water, which is advantageous for storing beverages and food. Glass packaging is sustainable, readily recycled, reusable and refillable.[133]
For electronics applications, glass can be used as a substrate in the manufacture of integrated passive devices, thin-film bulk acoustic resonators, and as a hermetic sealing material in device packaging,[134][135] including very thin solely glass based encapsulation of integrated circuits and other semiconductors in high manufacturing volumes.[136]
Laboratories
Glass is an important material in scientific laboratories for the manufacture of experimental apparatus because it is relatively cheap, readily formed into required shapes for experiment, easy to keep clean, can withstand heat and cold treatment, is generally non-reactive with many reagents, and its transparency allows for the observation of chemical reactions and processes.[137][138] Laboratory glassware applications include flasks, petri dishes, test tubes, pipettes, graduated cylinders, glass lined metallic containers for chemical processing, fractionation columns, glass pipes, Schlenk lines, gauges, and thermometers.[139][137] Although most standard laboratory glassware has been mass-produced since the 1920s, scientists still employ skilled glassblowers to manufacture bespoke glass apparatus for their experimental requirements.[140]
-
A Vigreux column in a laboratory setup
-
-
Optics
Glass is a ubiquitous material in optics by virtue of its ability to refract, reflect, and transmit light. These and other optical properties can be controlled by varying chemical compositions, thermal treatment, and manufacturing techniques. The many applications of glass in optics includes glasses for eyesight correction, imaging optics (e.g. lenses and mirrors in telescopes, microscopes, and cameras), fibre optics in telecommunications technology, and integrated optics. Microlenses and gradient-index optics (where the refractive index is non-uniform) find application in e.g. reading optical discs, laser printers, photocopiers, and laser diodes.[55]
Art
Glass as art dates to least 1300 BC shown as an example of natural glass found in Tutankhamun’s pectoral,[141] which also contained vitreous enamel, that is to say, melted coloured glass used on a metal backing. Enamelled glass, the decoration of glass vessels with coloured glass paints, has existed since 1300 BC,[142] and was prominent in the early 20th century with Art Nouveau glass and that of the House of Fabergé in St. Petersburg, Russia. Both techniques were used in stained glass, which reached its height roughly from 1000 to 1550, before a revival in the 19th century.
The 19th century saw a revival in ancient glassmaking techniques including cameo glass, achieved for the first time since the Roman Empire, initially mostly for pieces in a neo-classical style. The Art Nouveau movement made great use of glass, with René Lalique, Émile Gallé, and Daum of Nancy in the first French wave of the movement, producing coloured vases and similar pieces, often in cameo glass or in lustre glass techniques.[143]
Louis Comfort Tiffany in America specialised in stained glass, both secular and religious, in panels and his famous lamps. The early 20th-century saw the large-scale factory production of glass art by firms such as Waterford and Lalique. Small studios may hand-produce glass artworks. Techniques for producing glass art include blowing, kiln-casting, fusing, slumping, pâte de verre, flame-working, hot-sculpting and cold-working. Cold work includes traditional stained glass work and other methods of shaping glass at room temperature. Objects made out of glass include vessels, paperweights, marbles, beads, sculptures and installation art.[144]
-
Émile Gallé, Marquetry glass vase with clematis flowers (1890-1900)
-
-
-
A glass sculpture by Dale Chihuly, «The Sun» at the «Gardens of Glass» exhibition in Kew Gardens, London
See also
- Fire glass
- Flexible glass
- Kimberley points
- Prince Rupert’s drop
- Smart glass
References
- ^ ASTM definition of glass from 1945
- ^ a b Zallen, R. (1983). The Physics of Amorphous Solids. New York: John Wiley. pp. 1–32. ISBN 978-0-471-01968-8.
- ^ Cusack, N.E. (1987). The physics of structurally disordered matter: an introduction. Adam Hilger in association with the University of Sussex press. p. 13. ISBN 978-0-85274-829-9.
- ^ a b c Scholze, Horst (1991). Glass – Nature, Structure, and Properties. Springer. pp. 3–5. ISBN 978-0-387-97396-8.
- ^ a b c d Elliot, S.R. (1984). Physics of Amorphous Materials. Longman group ltd. pp. 1–52. ISBN 0-582-44636-8.
- ^ Neumann, Florin. «Glass: Liquid or Solid – Science vs. an Urban Legend». Archived from the original on 9 April 2007. Retrieved 8 April 2007.
- ^ a b c Gibbs, Philip. «Is glass liquid or solid?». Archived from the original on 29 March 2007. Retrieved 21 March 2007.
- ^ «Philip Gibbs» Glass Worldwide, (May/June 2007), pp. 14–18
- ^ Salmon, P.S. (2002). «Order within disorder». Nature Materials. 1 (2): 87–8. doi:10.1038/nmat737. PMID 12618817. S2CID 39062607.
- ^ Vannoni, M.; Sordini, A.; Molesini, G. (2011). «Relaxation time and viscosity of fused silica glass at room temperature». Eur. Phys. J. E. 34 (9): 9–14. doi:10.1140/epje/i2011-11092-9. PMID 21947892. S2CID 2246471.
- ^ Anderson, P.W. (1995). «Through the Glass Lightly». Science. 267 (5204): 1615–16. doi:10.1126/science.267.5204.1615-e. PMID 17808155. S2CID 28052338.
- ^ Phillips, J.C. (1979). «Topology of covalent non-crystalline solids I: Short-range order in chalcogenide alloys». Journal of Non-Crystalline Solids. 34 (2): 153. Bibcode:1979JNCS…34..153P. doi:10.1016/0022-3093(79)90033-4.
- ^ Folmer, J.C.W.; Franzen, Stefan (2003). «Study of polymer glasses by modulated differential scanning calorimetry in the undergraduate physical chemistry laboratory». Journal of Chemical Education. 80 (7): 813. Bibcode:2003JChEd..80..813F. doi:10.1021/ed080p813.
- ^ Loy, Jim. «Glass Is A Liquid?». Archived from the original on 14 March 2007. Retrieved 21 March 2007.
- ^ «Obsidian: Igneous Rock – Pictures, Uses, Properties». geology.com.
- ^ «Impactites: Impact Breccia, Tektites, Moldavites, Shattercones». geology.com.
- ^ Klein, Hermann Joseph (1 January 1881). Land, sea and sky; or, Wonders of life and nature, tr. from the Germ. [Die Erde und ihr organisches Leben] of H.J. Klein and dr. Thomé, by J. Minshull.
- ^ Giaimo, Cara (June 30, 2017). «The Long, Weird Half-Life of Trinitite». Atlas Obscura. Retrieved July 8, 2017.
- ^ Roperch, Pierrick; Gattacceca, Jérôme; Valenzuela, Millarca; Devouard, Bertrand; Lorand, Jean-Pierre; Arriagada, Cesar; Rochette, Pierre; Latorre, Claudio; Beck, Pierre (2017). «Surface vitrification caused by natural fires in Late Pleistocene wetlands of the Atacama Desert». Earth and Planetary Science Letters. 469 (1 July 2017): 15–26. Bibcode:2017E&PSL.469…15R. doi:10.1016/j.epsl.2017.04.009. S2CID 55581133.
- ^ a b c d e f Ward-Harvey, K. (2009). Fundamental Building Materials. Universal-Publishers. pp. 83–90. ISBN 978-1-59942-954-0.
- ^ «Digs Reveal Stone-Age Weapons Industry With Staggering Output». National Geographic News. 13 April 2015.
- ^ a b c Julian Henderson (2013). Ancient Glass. Cambridge University Press. pp. 127–157. doi:10.1017/CBO9781139021883.006.
- ^ «Glass Online: The History of Glass». Archived from the original on 24 October 2011. Retrieved 29 October 2007.
- ^ «All About Glass | Corning Museum of Glass». www.cmog.org.
- ^ Karklins, Karlis (January 2013). «Simon Kwan – Early Chinese Faience and Glass Beads and Pendants». BEADS: Journal of the Society of Bead Researchers.
- ^ Kenoyer, J.M (2001). «Bead Technologies at Harappa, 3300-1900 BC: A Comparative Summary». South Asian Archaeology (PDF). Paris. pp. 157–170. Archived (PDF) from the original on 8 July 2019.
- ^ McIntosh, Jane (2008). The Ancient Indus Valley: New Perspectives. ABC-CLIO. p. 99. ISBN 978-1-57607-907-2.
- ^ «How did Manufactured Glass Develop in the Bronze Age? — DailyHistory.org». dailyhistory.org.
- ^ Wilde, H. «Technologische Innovationen im 2. Jahrtausend v. Chr. Zur Verwendung und Verbreitung neuer Werkstoffe im ostmediterranen Raum». GOF IV, Bd 44, Wiesbaden 2003, 25–26.
- ^ Douglas, R.W. (1972). A history of glassmaking. Henley-on-Thames: G T Foulis & Co Ltd. p. 5. ISBN 978-0-85429-117-5.
- ^ Whitehouse, David (2003). Roman Glass in the Corning Museum of Glass, Volume 3. Hudson Hills. p. 45. ISBN 978-0-87290-155-1.
- ^ The Art Journal. Virtue and Company. 1888. p. 365.
- ^ Brown, A.L. (November 1921). «The Manufacture of Glass Milk Bottles». The Glass Industry. Ashlee Publishing Company. 2 (11): 259.
- ^ Aton, Francesca, Perfectly Preserved 2,000-Year-Old Roman Glass Bowl Unearthed in the Netherlands, Art News, January 25, 2022
- ^ McGreevy, Nora, 2,000-Year-Old Roman Bowl Discovered Intact in the Netherlands, National Geographic, January 28, 2022
- ^ Dien, Albert E. (2007). Six Dynasties Civilization. Yale University Press. p. 290. ISBN 978-0-300-07404-8.
- ^ Silberman, Neil Asher; Bauer, Alexander A. (2012). The Oxford Companion to Archaeology. Oxford University Press. p. 29. ISBN 978-0-19-973578-5.
- ^ a b c d «glass | Definition, Composition, & Facts». Encyclopedia Britannica.
- ^ Oliver, Roland, and Fagan, Brian M. Africa in the Iron Age, c500 B.C. to A.D. 1400. New York: Cambridge University Press, p. 187. ISBN 0-521-20598-0.
- ^ Keller, Daniel; Price, Jennifer; Jackson, Caroline (2014). Neighbours and Successors of Rome: Traditions of Glass Production and use in Europe and the Middle East in the Later 1st Millennium AD. Oxbow Books. pp. 1–41. ISBN 978-1-78297-398-0.
- ^ Tutag, Nola Huse; Hamilton, Lucy (1987). Discovering Stained Glass in Detroit. Wayne State University Press. pp. 11. ISBN 978-0-8143-1875-1.
- ^ Packard, Robert T.; Korab, Balthazar; Hunt, William Dudley (1980). Encyclopedia of American architecture. McGraw-Hill. pp. 268. ISBN 978-0-07-048010-0.
- ^ a b
One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). «Glass». Encyclopædia Britannica. Vol. 12 (11th ed.). Cambridge University Press. p. 86.
- ^ Freiman, Stephen (2007). Global Roadmap for Ceramic and Glass Technology. John Wiley & Sons. p. 705. ISBN 978-0-470-10491-0.
- ^ «Depression Glass». Archived from the original on 2 December 2014. Retrieved 19 October 2007.
- ^ Gelfand, Lisa; Duncan, Chris (2011). Sustainable Renovation: Strategies for Commercial Building Systems and Envelope. John Wiley & Sons. p. 187. ISBN 978-1-118-10217-6.
- ^ Lim, Henry W.; Honigsmann, Herbert; Hawk, John L.M. (2007). Photodermatology. CRC Press. p. 274. ISBN 978-1-4200-1996-4.
- ^ Bach, Hans; Neuroth, Norbert (2012). The Properties of Optical Glass. Springer. p. 267. ISBN 978-3-642-57769-7.
- ^ McLean, Ian S. (2008). Electronic Imaging in Astronomy: Detectors and Instrumentation. Springer Science & Business Media. p. 78. ISBN 978-3-540-76582-0.
- ^ a b c «Glass Applications – Glass Alliance Europe». Glassallianceeurope.eu. Retrieved 1 March 2020.
- ^ Enteria, Napoleon; Akbarzadeh, Aliakbar (2013). Solar Energy Sciences and Engineering Applications. CRC Press. p. 122. ISBN 978-0-203-76205-9.
- ^ «Gorilla Glass maker unveils ultra-thin and flexible Willow Glass». Physics News. Archived from the original on 6 November 2013. Retrieved 1 November 2013.
- ^ «Xensation». Schott. Archived from the original on 3 November 2013. Retrieved 1 November 2013.
- ^ Fingas, Jon (19 July 2018). «Gorilla Glass 6 gives phones a better shot at surviving multiple drops». Engadget.
- ^ a b c d Bach, Hans; Neuroth, Norbert (2012). The Properties of Optical Glass. Springer. pp. 1–11. ISBN 978-3-642-57769-7.
- ^ White, Mary Anne (2011). Physical Properties of Materials, Second Edition. CRC Press. p. 70. ISBN 978-1-4398-9532-0.
- ^ Carter, C. Barry; Norton, M. Grant (2007). Ceramic Materials: Science and Engineering. Springer Science & Business Media. p. 583. ISBN 978-0-387-46271-4.
- ^ Mysen, Bjorn O.; Richet, Pascal (2005). Silicate Glasses and Melts: Properties and Structure. Elsevier. p. 10.
- ^ a b c d e «Industrial glass – Properties of glass». Encyclopedia Britannica.
- ^ Mattox, D.M. (2014). Handbook of Physical Vapor Deposition (PVD) Processing. Cambridge University Press. p. 60. ISBN 978-0-08-094658-0.
- ^ Zarzycki, Jerzy (1991). Glasses and the Vitreous State. Cambridge University Press. p. 361. ISBN 978-0-521-35582-7.
- ^ Thomas, Alfred; Jund, Michael (2013). Collision Repair and Refinishing: A Foundation Course for Technicians. p. 365. ISBN 978-1-133-60187-6.
- ^ a b Gardner, Irvine Clifton; Hahner, Clarence H. (1949). Research and Development in Applied Optics and Optical Glass at the National Bureau of Standards: A Review and Bibliography. U.S. Government Printing Office. p. 13. ISBN 9780598682413.
- ^ Dudeja, Puja; Gupta, Rajul K.; Minhas, Amarjeet Singh (2016). Food Safety in the 21st Century: Public Health Perspective. Academic Press. p. 550. ISBN 978-0-12-801846-0.
- ^ Bengisu, M. (2013). Engineering Ceramics. Springer Science & Business Media. p. 360. ISBN 978-3-662-04350-9.
- ^ Batchelor, Andrew W.; Loh, Nee Lam; Chandrasekaran, Margam (2011). Materials Degradation and Its Control by Surface Engineering. World Scientific. p. 141. ISBN 978-1-908978-14-1.
- ^ a b c d Chawla, Sohan L. (1993). Materials Selection for Corrosion Control. ASM International. pp. 327–328. ISBN 978-1-61503-728-5.
- ^ Shaye Storm (2004). «Density of Glass». The Physics Factbook: An encyclopedia of scientific essays. Wikidata Q87511351.
- ^ «Glass Strength». www.pilkington.com. Archived from the original on 26 July 2017. Retrieved 24 November 2017.
- ^ Kenneth Chang (29 July 2008). «The Nature of Glass Remains Anything but Clear». The New York Times. Archived from the original on 24 April 2009. Retrieved 29 July 2008.
- ^ Gulbiten, Ozgur; Mauro, John C.; Guo, Xiaoju; Boratav, Olus N. (3 August 2017). «Viscous flow of medieval cathedral glass». Journal of the American Ceramic Society. 101 (1): 5–11. doi:10.1111/jace.15092. ISSN 0002-7820.
- ^ Gocha, April (3 August 2017). «Glass viscosity calculations definitively debunk the myth of observable flow in medieval windows». The American Ceramic Society.
- ^ a b c «Mining the sea sand». Seafriends. 8 February 1994. Archived from the original on 29 February 2012. Retrieved 15 May 2012.
- ^ a b c d e f «Glass – Chemistry Encyclopedia». Archived from the original on 2 April 2015. Retrieved 1 April 2015.
- ^ a b B.H.W.S. de Jong, «Glass»; in «Ullmann’s Encyclopedia of Industrial Chemistry»; 5th edition, vol. A12, VCH Publishers, Weinheim, Germany, 1989, ISBN 978-3-527-20112-9, pp. 365–432.
- ^ a b Spence, William P.; Kultermann, Eva (2016). Construction Materials, Methods and Techniques. Cengage Learning. pp. 510–526. ISBN 978-1-305-08627-2.
- ^ «Properties of PYREX®, PYREXPLUS® and Low Actinic PYREX Code 7740 Glasses» (PDF). Corning, Inc. Archived (PDF) from the original on 13 January 2012. Retrieved 15 May 2012.
- ^ «AR-GLAS® Technical Data» (PDF). Schott, Inc. Archived (PDF) from the original on 12 June 2012.
- ^ Shelby, J.E. (2017). Introduction to Glass Science and Technology. Royal Society of Chemistry. p. 125. ISBN 978-0-85404-639-3.
- ^ Schwartz, Mel (2002). Encyclopedia of Materials, Parts and Finishes (Second ed.). CRC Press. p. 352. ISBN 978-1-4200-1716-8.
- ^ Shackelford, James F.; Doremus, Robert H. (12 April 2008). Ceramic and Glass Materials: Structure, Properties and Processing. Springer Science & Business Media. p. 158. ISBN 978-0-387-73362-3.
- ^ Askeland, Donald R.; Fulay, Pradeep P. (2008). Essentials of Materials Science & Engineering. Cengage Learning. p. 485. ISBN 978-0-495-24446-2.
- ^ «Glass Ingredients – What is Glass Made Of?». www.historyofglass.com. Archived from the original on 23 April 2017. Retrieved 23 April 2017.
- ^ Pfaender, Heinz G. (1996). Schott guide to glass. Springer. pp. 135, 186. ISBN 978-0-412-62060-7. Archived from the original on 25 May 2013. Retrieved 8 February 2011.
- ^ Doering, Robert; Nishi, Yoshio (2007). Handbook of semiconductor manufacturing technology. CRC Press. pp. 12–13. ISBN 978-1-57444-675-3.
- ^ a b c Holand, Wolfram; Beall, George H. (2012). Glass Ceramic Technology. John Wiley & Sons. pp. 1–38. ISBN 978-1-118-26592-5.
- ^ Richerson, David W. (1992). Modern ceramic engineering : properties, processing and use in design (2nd ed.). New York: Dekker. pp. 577–578. ISBN 978-0-8247-8634-2.
- ^ a b Parkyn, Brian (2013). Glass Reinforced Plastics. Elsevier. pp. 3–41. ISBN 978-1-4831-0298-6.
- ^ Mayer, Rayner M. (1993). Design with reinforced plastics. Springer. p. 7. ISBN 978-0-85072-294-9.
- ^ a b «Properties of Matter Reading Selection: Perfect Teamwork». www.propertiesofmatter.si.edu. Archived from the original on 12 May 2016. Retrieved 25 April 2017.
- ^ a b «Fibreglass | glass». Encyclopedia Britannica.
- ^ Greer, A. Lindsay; Mathur, N (2005). «Materials science: Changing Face of the Chameleon». Nature. 437 (7063): 1246–1247. Bibcode:2005Natur.437.1246G. doi:10.1038/4371246a. PMID 16251941. S2CID 6972351.
- ^ Rivera, V. A. G.; Manzani, Danilo (30 March 2017). Technological Advances in Tellurite Glasses: Properties, Processing, and Applications. Springer. p. 214. ISBN 978-3-319-53038-3.
- ^ Jiang, Xin; Lousteau, Joris; Richards, Billy; Jha, Animesh (1 September 2009). «Investigation on germanium oxide-based glasses for infrared optical fibre development». Optical Materials. 31 (11): 1701–1706. Bibcode:2009OptMa..31.1701J. doi:10.1016/j.optmat.2009.04.011.
- ^ J. W. E. Drewitt; S. Jahn; L. Hennet (2019). «Configurational constraints on glass formation in the liquid calcium aluminate system». Journal of Statistical Mechanics: Theory and Experiment. 2019 (10): 104012. arXiv:1909.07645. Bibcode:2019JSMTE..10.4012D. doi:10.1088/1742-5468/ab47fc. S2CID 202583753.
- ^ C. J. Benmore; J. K. R. Weber (2017). «Aerodynamic levitation, supercooled liquids and glass formation». Advances in Physics: X. 2 (3): 717–736. Bibcode:2017AdPhX…2..717B. doi:10.1080/23746149.2017.1357498.
- ^ Davies, H. A.; Hull J. B. (1976). «The formation, structure and crystallization of non-crystalline nickel produced by splat-quenching». Journal of Materials Science. 11 (2): 707–717. Bibcode:1976JMatS..11..215D. doi:10.1007/BF00551430. S2CID 137403190.
- ^ Klement, W. Jr.; Willens, R.H.; Duwez, Pol (1960). «Non-crystalline Structure in Solidified Gold-Silicon Alloys». Nature. 187 (4740): 869. Bibcode:1960Natur.187..869K. doi:10.1038/187869b0. S2CID 4203025.
- ^ Liebermann, H.; Graham, C. (1976). «Production of Amorphous Alloy Ribbons and Effects of Apparatus Parameters on Ribbon Dimensions». IEEE Transactions on Magnetics. 12 (6): 921. Bibcode:1976ITM….12..921L. doi:10.1109/TMAG.1976.1059201.
- ^ Ponnambalam, V.; Poon, S. Joseph; Shiflet, Gary J. (2004). «Fe-based bulk metallic glasses with diameter thickness larger than one centimeter». Journal of Materials Research. 19 (5): 1320. Bibcode:2004JMatR..19.1320P. doi:10.1557/JMR.2004.0176.
- ^ «Metallurgy Division Publications». NIST Interagency Report 7127. Archived from the original on 16 September 2008.
- ^ Mendelev, M.I.; Schmalian, J.; Wang, C.Z.; Morris, J.R.; K.M. Ho (2006). «Interface Mobility and the Liquid-Glass Transition in a One-Component System». Physical Review B. 74 (10): 104206. Bibcode:2006PhRvB..74j4206M. doi:10.1103/PhysRevB.74.104206.
- ^ «A main research field: Polymer glasses». www-ics.u-strasbg.fr. Archived from the original on 25 May 2016.
- ^ Carraher, Charles E. Jr. (2012). Introduction to Polymer Chemistry. CRC Press. p. 274. ISBN 978-1-4665-5495-5.
- ^ Ruby, S.L.; Pelah, I. (2013). «Crystals, Supercooled Liquids, and Glasses in Frozen Aqueous Solutions». In Gruverman, Irwin J. (ed.). Mössbauer Effect Methodology: Volume 6 Proceedings of the Sixth Symposium on Mössbauer Effect Methodology New York City, January 25, 1970. Springer Science & Business Media. p. 21. ISBN 978-1-4684-3159-9.
- ^ Levine, Harry; Slade, Louise (2013). Water Relationships in Foods: Advances in the 1980s and Trends for the 1990s. Springer Science & Business Media. p. 226. ISBN 978-1-4899-0664-9.
- ^ Dupuy J, Jal J, Prével B, Aouizerat-Elarby A, Chieux P, Dianoux AJ, Legrand J (October 1992). «Vibrational dynamics and structural relaxation in aqueous electrolyte solutions in the liquid, undercooled liquid and glassy states» (PDF). Journal de Physique IV. 2 (C2): C2-179–C2-184. Bibcode:1992JPhy4…2C.179D. doi:10.1051/jp4:1992225. S2CID 39468740. Archived (PDF) from the original on 9 May 2020. European Workshop on Glasses and Gels.
- ^ Hartel, Richard W.; Hartel, AnnaKate (2014). Candy Bites: The Science of Sweets. Springer Science & Business Media. p. 38. ISBN 978-1-4614-9383-9.
- ^ Charbel Tengroth (2001). «Structure of Ca0.4K0.6(NO3)1.4 from the glass to the liquid state». Phys. Rev. B. 64 (22): 224207. Bibcode:2001PhRvB..64v4207T. doi:10.1103/PhysRevB.64.224207.
- ^ «Lithium-Ion Pioneer Introduces New Battery That’s Three Times Better». Fortune. Archived from the original on 9 April 2017. Retrieved 6 May 2017.
- ^ «PFG Glass». Pfg.co.za. Archived from the original on 6 November 2009. Retrieved 24 October 2009.
- ^ Code of Federal Regulations, Title 40,: Protection of Environment, Part 60 (Sections 60.1-end), Revised As of July 1, 2011. Government Printing Office. October 2011. ISBN 978-0-16-088907-3.
- ^ Ball, Douglas J.; Norwood, Daniel L.; Stults, Cheryl L. M.; Nagao, Lee M. (24 January 2012). Leachables and Extractables Handbook: Safety Evaluation, Qualification, and Best Practices Applied to Inhalation Drug Products. John Wiley & Sons. p. 552. ISBN 978-0-470-17365-7.
- ^ Chisholm, Hugh, ed. (1911). «Glass» . Encyclopædia Britannica. Vol. 12 (11th ed.). Cambridge University Press. pp. 87–105.
- ^ «windshields how they are made». autoglassguru. Retrieved 9 February 2018.
- ^ Pantano, Carlo. «Glass Surface Treatments: Commercial Processes Used in Glass Manufacture» (PDF). Archived (PDF) from the original on 9 September 2015.
- ^ a b «Glass melting, Pacific Northwest National Laboratory». Depts.washington.edu. Archived from the original on 5 May 2010. Retrieved 24 October 2009.
- ^ Fluegel, Alexander. «Glass melting in the laboratory». Glassproperties.com. Archived from the original on 13 February 2009. Retrieved 24 October 2009.
- ^ a b c d e f Mukherjee, Swapna (2013). The Science of Clays: Applications in Industry, Engineering, and Environment. Springer Science & Business Media. p. 142. ISBN 978-9-4007-6683-9.
- ^ Walker, Perrin; Tarn, William H. (1990). CRC Handbook of Metal Etchants. CRC press. p. 798. ISBN 978-1-4398-2253-1.
- ^ a b Langhamer, Antonín (2003). The Legend of Bohemian Glass: A Thousand Years of Glassmaking in the Heart of Europe. Tigris. p. 273. ISBN 978-8-0860-6211-2.
- ^ «3. Glass, Colour and the Source of Cobalt». Internet Archaeology.
- ^ Chemical Fact Sheet – Chromium Archived 2017-08-15 at the Wayback Machine www.speclab.com.
- ^ David M Issitt. Substances Used in the Making of Coloured Glass 1st.glassman.com.
- ^ Shelby, James E. (2007). Introduction to Glass Science and Technology. Royal Society of Chemistry. p. 211. ISBN 978-1-84755-116-0.
- ^ a b Nicholson, Paul T.; Shaw, Ian (2000). Ancient Egyptian Materials and Technology. Cambridge University Press. p. 208. ISBN 978-0-521-45257-1.
- ^ Weller, Bernhard; Unnewehr, Stefan; Tasche, Silke; Härth, Kristina (2012). Glass in Building: Principles, Applications, Examples. Walter de Gruyter. pp. 1–19. ISBN 978-3-0346-1571-6.
- ^ a b «The rise of glass buildings». Glass Times. 9 January 2017. Retrieved 1 March 2020.
- ^ Patterson, Mic (2011). Structural Glass Facades and Enclosures. Jon Wiley & Sons. p. 29. ISBN 978-0-470-93185-1.
- ^ Hynes, Michael; Jonson, Bo (1997). «Lead, glass and the environment». Chemical Society Reviews. 26 (2): 145. doi:10.1039/CS9972600133.
- ^ «Cut glass | decorative arts». Encyclopedia Britannica.
- ^ «High temperature glass melt property database for process modeling»; Eds.: Thomas P. Seward III and Terese Vascott; The American Ceramic Society, Westerville, Ohio, 2005, ISBN 1-57498-225-7
- ^ «Why choose Glass?». FEVE.
- ^ Sun, P.; et, al. (2018). «Design and Fabrication of Glass-based Integrated Passive Devices». IEEE, 19th International Conference on Electronic Packaging Technology (ICEPT): 59–63. doi:10.1109/ICEPT.2018.8480458. ISBN 978-1-5386-6386-8. S2CID 52935909.
- ^ Letz, M.; et, al. (2018). «Glass in Electronic Packaging and Integration: High Q Inductances for 2.35 GHz Impedance Matching in 0.05 mm Thin Glass Substrates». IEEE 68th Electronic Components and Technology Conference (ECTC): 1089–1096. doi:10.1109/ECTC.2018.00167. ISBN 978-1-5386-4999-2. S2CID 51972637.
- ^ Lundén, H.; et, al. (2004). «Novel glass welding technique for hermetic encapsulation». Proceedings of the 5th Electronics System-integration Technology Conference (ESTC): 1–4. doi:10.1109/ESTC.2014.6962719. ISBN 978-1-4799-4026-4. S2CID 9980556.
- ^ a b Zumdahl, Steven (2013). Lab Manual. Cengage Learning. pp. ix–xv. ISBN 978-1-285-69235-7.
- ^ «Science Under Glass». National Museum of American History. 29 July 2015.
- ^ Basudeb, Karmakar (2017). Functional Glasses and Glass-Ceramics: Processing, Properties and Applications. Butterworth-Heinemann. pp. 3–5. ISBN 978-0-12-805207-5.
- ^ «Scientific Glassblowing | National Museum of American History». Americanhistory.si.edu. 17 December 2012. Retrieved 4 March 2020.
- ^ Tut’s gem hints at space impact, BBC News, July 19, 2006.
- ^ The Earliest Cloisonné Enamels
- ^ Arwas, Victor (1996). The Art of Glass: Art Nouveau to Art Deco. pp. 1–54. ISBN 978-1-901092-00-4.
- ^ «A-Z of glass». Victoria and Albert Museum. Retrieved 9 March 2020.
External links
- «Glass» . Encyclopædia Britannica. Vol. 12 (11th ed.). 1911.
- The Story of Glass Making in Canada from The Canadian Museum of Civilization.
- «How Your Glass Ware Is Made» by George W. Waltz, February 1951, Popular Science.
- All About Glass from the Corning Museum of Glass: a collection of articles, multimedia, and virtual books all about glass, including the Glass Dictionary.
- National Glass Association—The largest trade association representing the flat (architectural), auto glass, and window & door industries
-
#1
Hi,
Could you see the picture above please? This is a glass (container). It is made of glass (material).
Can I say:
This glass is of glass.
Or,
This is a glass of glass.
[In these sentences, the first «glass» is the container, the second «glass» is the material]
-
#2
No. But you could say it was made of glass.
-
#3
It is possible to have a plastic glass. To distinguish this from a
real glass glass
, we might also say that. It’s an informal, rather jokey way, but we would say that, not ‘glass of glass’.
-
#4
In the same manner, would you also not say «glass of plastic»?
-
#5
In the same manner, would you also not say «glass of plastic»?
In the manner that we don’t say «glass of glass» (we say «glass»), we also don’t say «glass of plastic» (we say «plastic glass»).
-
#6
Again, we’d describe it as
made of
plastic, not just
of
plastic. The phrase “glass of plastic” would be possible in the right context, but not just as a basic description of the item.
Last edited: Jan 11, 2022
-
#7
We sell three types of drinking glasses: We have plastic glasses, glass glasses, and glass stem glasses.
This works because of the parallel structure of «plastic glasses» and «glass glasses»; otherwise it would be odd sounding.
-
#8
I would view a «glass» such as the one depicted as made of glass by default.
To me, a drinking receptacle made of plastic is a «plastic cup», even if it is shaped like a drinking glass. I couldn’t imagine saying it was a «plastic glass».
-
#9
The phrase «a glass of X» means a glass containing X (milk, water, beer, tea, etc.). This «of» is not the material the container is made out of (glass). It is the material that is contained (milk, water, etc.)
This is a very common use of «of» in English. We turn an uncountable substance (water, milk) into a countable phrase using:
— a container of substance
— a quantity of substance
For example, «soup, water, petrol, sand, sugar» are all uncountable substances. These are all countable noun phrases:
— a glass of milk
— a can of petrol
— a bowl of soup
— a liter of petrol
— a liter of sand
— a bucket of sand
— a cup of sugar
elroy
Moderator: EHL, Arabic, Hebrew, German(-Spanish)
-
#10
I agree with @anthox. I would not use «glass» to refer to a drinking vessel that was
not
made of glass.
-
#11
I would view a «glass» such as the one depicted as made of glass by default.
To me, a drinking receptacle made of plastic is a «plastic cup», even if it is shaped like a drinking glass. I couldn’t imagine saying it was a «plastic glass».
In my mind, a plastic cup is not the same as a glass made from plastic.
This from the Webstaurant website:
Whether you are serving wine on the patio or by the pool, this GET SW-1404-CL plastic wine glass is a great way to reduce breakage and protect your guests against broken glass while still offering a quality presentation. You could even use it at your next catered event or party for carefree, showstopping service! Whether you’re serving white wine, red, or champagne, this plastic wine glass is sure to please.
-
#12
I agree with @anthox. I would not use «glass» to refer to a drinking vessel that was
not
made of glass.
If you ask for a glass of water and they bring you a clear, sturdy, plastic container that looks like a glass made of glass in every way, do you send it back? I’m very careful now with the water glasses in restaurants. I came close to throwing one up in the air one time because it wasn’t as heavy as it looked due to being made of plastic.
elroy
Moderator: EHL, Arabic, Hebrew, German(-Spanish)
-
#13
No, because in that context I don’t actually care what it’s made of: «a glass of water» isn’t meant to be taken ultra-literally in that context.
-
#14
I could use «a glass glass» vs. «a plastic glass».
The phrase «a glass of X» means a glass containing X (milk, water, beer, tea, etc.). This «of» is not the material the container is made out of (glass). It is the material that is contained (milk, water, etc.)
This is a very common use of «of» in English. We turn an uncountable substance (water, milk) into a countable phrase using:
— a container of substance
— a quantity of substanceFor example, «soup, water, petrol, sand, sugar» are all uncountable substances. These are all countable noun phrases:
— a glass of milk
— a can of petrol
— a bowl of soup
— a liter of petrol
— a liter of sand
— a bucket of sand
— a cup of sugar
Yes, for instance
«a matchbox»
/»a wine glass»/»a soup bowl» for the container,
«a box of matches»
/»a glass of wine»/»a bowl of soup» for the quantity/number of the contents.
On second thought, «matchbox» doesn’t belong here; it’s one word.
-
#15
A plastic vessel made for taking on picnics is just a plastic something, but some plastic vessels are specifically made as substitutes for (or copies of) glasses. A pub stocks very definite legal sizes of glass, and if they run out of glasses, they may have a box of plastic ones of the same size and shape for temporary use. These would be called plastic glasses.
Usage changes with times. Corks in wine bottles used to be made out of cork. Now we distinguish plastic corks from real corks — cork corks?
-
#16
You could say «This glass is actually made of glass».
I have very often come across the expression «plastic glasses», and I once knew a woman who said «I absolutely refuse to drink wine from plastic glasses».
-
#17
The best general rule to stick to is to avoid «thing of substance», and use «thing made of substance» or «substance thing» instead.
Not a shirt of silk… a shirt made of silk
… or a silk shirt
Not a pipe of copper… a pipe made of copper
… or a copper pipe
Not a driveway of concrete… a driveway made of concrete
… or a concrete driveway
Not a bowl of stainless steel… a bowl made of stainless steel
… or a stainless-steel bowl
Not insulation of fiberglass/rockwool… insulation made of fiberglass/rockwool
… or fiberglass/rockwool insulation
Not a can of tin/aluminum… a can made of tin/aluminum
… or a tin/aluminum can
Not a coat of leather… a coat made of leather
… or a leather coat
In all cases, the one with the green checkmark, «substance thing», is the most common, and the best in most situations. «Thing made of substance» (the ones I marked with a light-bulb above) is technically a correct option but not used as much. At this moment, the only situation I can think of in which I’d use it is if I were comparing alternative substances to make a thing of, to teach somebody the benefits and drawbacks of each choice. And even then I’d probably still mix some «substance thing» formations in with those anyway.
Two substances, wood and gold, still use an otherwise archaic suffix to turn them into adjectives for «substance thing», making them «wooden» and «golden». A wooden table is a table made of wood (no suffix in «thing made of substance because it’s a noun there). I’ve also heard «woolen mittens» for mittens made of wool, but that was only in a song from the 1950s. Right now I can’t think of another noun that still uses the «-en» suffix. The rest can be used as an adjective or as a noun without changing the word. (Just imagine trying to say «aluminumen»!)
I have actually seen and heard «thing of substance» in some specific phrases, but the examples I can think of are all metaphors. In «bed of roses» and «heart of ice/stone», the bed isn’t really a bed, the ice or stone isn’t really ice or stone, the heart isn’t really a heart, and even the roses aren’t roses (they’re rose petals). When a chef talks about serving something «on a bed of rice», the bed isn’t really a bed; it just means some other food was put on top of rice. In «nerves of steel», the steel isn’t really steel, and the nerves aren’t necessarily nerves.
-
#18
< —— >
I happily use the terms «glass glass» and «plastic glass» when I want to refer to the material, and so does everyone I know. When you go to a pub that only allows plastic glasses to be used outside, it is common for both customers and the bar staff to refer to glasses made of glass and glasses made of plastic in this way.
< Topic drift removed. Cagey, moderator >
Last edited by a moderator: Jan 13, 2022
Glass can be made transparent and flat, or into other shapes and colors as shown in this ball from the Verrerie of Brehat in Brittany
From tiny beads to large sculptures, and from ordinary bottles to sophisticated lenses and optical fibers, the multiple uses of glass have transformed our world. The word glass may be defined as an amorphous solid that is usually produced by mixing silica with other chemicals at high heat, and allowing the mixture to cool without forming a crystalline structure. It is commonly a transparent, hardwearing, chemically inert, and biologically inactive material, with smooth, impervious surfaces. It is, however, brittle and can break into sharp shards. Glass-making technologies have developed over many centuries, and the properties of glass can be modified significantly with the addition of various compounds, heat treatment, and other techniques. Today, glass continues to be used extensively for both artistic expression and practical applications ranging from micro-pipettes in the microbiology laboratory to cookware in the home kitchen and from automobile windshields to lenses for telescopes.
History of glass
Glass has been considered a valuable material since early in human history. During the Stone Age, flint knappers used obsidian, a naturally occurring glass, to make extremely sharp knives. In addition, in early civilizations, small pieces of colored glass often rivaled precious gems as jewelry items. With the passage of time, it was discovered that if glass is heated until it becomes semi-liquid, it could be shaped and left to cool in a new, solid, independently standing shape.
Instructions to make glass were first documented in Egypt around 1500 B.C.E., when glass was used as a glaze for ceramic items before they were fired. In the first century B.C.E., the technique of blowing glass was developed, and what had once been an extremely rare and valuable item became much more common. At the time of the Roman Empire, many forms of glass were created, usually for vases and bottles. The glass was made from sand, plant ash, and lime (calcium oxide).
The color of «natural glass» usually varies between green and bluish green, based on the differing amounts of naturally occurring «impurities» of iron in the sand. Even today, common glass usually has a slight green or blue tint, arising from the same types of impurities. By contrast, obsidian, which is produced from volcanic magma, contains impurities that give it a black color.
Glassmakers learned to make colored glass by adding metallic compounds and mineral oxides to produce brilliant hues of red, green, and blue—the colors of gemstones (see Ingredients for colors below). When gem-cutters learned to cut glass, they found that clear glass was an excellent refractor of light, causing the glass to sparkle. Consequently, the popularity of cut clear glass soared, while that of colored glass diminished.
Glass objects from the seventh and eighth centuries have been found on the island of Torcello near Venice. These items form an important link between the Roman era and the later period, when that city gained importance in the production of the material. About 1000 C.E., an important technical breakthrough was made in Northern Europe, when soda glass (containing sodium carbonate) was replaced by glass made from a much more readily available material: potash (potassium carbonate), obtained from wood ashes. From this point on, northern glass differed significantly from that produced in the Mediterranean area, where soda remained in common use.
The eleventh century saw the emergence, in Germany, of a new approach to making sheet glass. The process involved blowing the glass into spheres, swinging them out to form cylinders, cutting them while still hot, and then flattening the sheets. This technique was perfected in thirteenth-century Venice.
From the fourteenth century onward, the center for glass making was Venice, where many new techniques were developed. Venice became the hub of a lucrative export trade in dinnerware, mirrors, and other luxury items. Eventually, some of the Venetian glassworkers moved to other parts of northern Europe, and glass making spread with them.
Around 1688, a process for casting glass was developed, and the material became much more commonly used. The glass-pressing machine was invented in 1827, allowing the mass production of inexpensive glass articles.
Artistic patterns are sometimes etched into glass using an acid or other caustic substance (which «eats» into the glass). Traditionally, this was done by a trained artisan after the glass was blown or cast. In the 1920s, a new mold-etch process was invented, by which an image was etched directly into the mold, so that each cast piece emerged from the mold with the image already on the surface of the glass. This technique reduced manufacturing costs and, combined with a wider use of colored glass, led to cheap, popular glassware in the 1930s, later known as «Depression glass.»
Glass-making processes
Hand-blown glass-making
Hand-blown glass beads and pendants. (A Canadian nickel is added for scale.)
Prior to the twentieth century, various methods of making hand-blown glass were developed. Some of the different types of glass produced by these processes were known as crown glass, broad sheet, cylinder blown sheet, blown plate, and polished plate.
The process for making crown glass was first perfected by French glassmakers in the 1320s, notably around Rouen. In this process, glass was blown into a «crown» or hollow globe, which was then reheated and spun out into a flat disk, up to 5–6 feet (1.5–1.8 meters) in diameter. Because of the manufacturing process, the best and thinnest glass would lie in a band at the edge of the disk, while the glass became thicker and more distorted toward the center. To fill large window spaces, many small diamond-shaped pieces would be cut from the edge of the disk, mounted into a lead latticework, and fitted in the window. The process was kept a careful trade secret for centuries. As a result, crown glass was not made in London until 1678.
Broad sheet glass was made by blowing molten glass into an elongated balloon shape with a blowpipe. Then, while the glass was still hot, the ends were cut off and the resulting cylinder was split with shears and flattened on an iron plate. The quality of broad sheet glass was not good, with many imperfections. Cylinder blown sheet was created using a similar process, but a larger cylinder was made by swinging it in a trench. The process produced much larger panes and improved surface quality over broad sheet. Blown plate was made from broad sheet glass by laboriously hand grinding and polishing both surfaces. It was of sufficient quality and size for mirrors and coach glasses.
Machine-manufacturing processes
The early twentieth century marked the move away from hand-blown to machine-manufactured glass, such as machine drawn cylinder sheet, rolled plate, and float glass.
Machine drawn cylinder sheet was the first mechanical method for «drawing» window glass. Cylinders of glass 40 feet (12 meters) high are drawn vertically from a circular tank. The glass is then annealed (held at a temperature at which internal stresses are relieved) and cut into 7–10 foot (2–3 meter) cylinders. The cylinders are cut lengthwise, reheated, and flattened. This process was invented in the United States in 1903.
In the rolled plate process, molten glass is taken from a furnace in large iron ladles, which are carried on slings running on overhead rails. The material in the ladle is thrown on the cast-iron bed of a rolling table and rolled into a sheet by an iron roller. The sheet is roughly trimmed while hot, to remove portions spoilt by contact with the ladle, and then pushed into the open mouth of an annealing tunnel or «lehr,» down which it is carried by a system of rollers.
To make float glass, several raw materials—sand, limestone (calcium carbonate), soda ash (sodium carbonate), dolomite (calcium magnesium carbonate), iron oxide, and salt cake (sodium sulfate)—are mixed together and melted in a large furnace. This material is poured onto one end of a molten tin bath. The glass floats on the tin and levels out as it spreads over the bath, giving a smooth glossy face to both sides. As it travels over the tin, the glass cools, solidifies, and leaves the bath in a continuous ribbon. The glass is then annealed in a lehr. The finished product has near-perfect parallel surfaces. This process produces about 90 percent of the world’s flat glass.
Properties of glass
One of the most obvious characteristics of ordinary glass is that it is transparent to visible light. It is therefore useful for making windows and various see-through objects. By varying its composition, texture, and color, glass can be made translucent or opaque.
Glass is chemically stable and unreactive, and retains its shape and structural integrity at ordinary temperatures for long periods. In addition, it can be readily cleaned with regular soaps and detergents. These properties make it a good material for containers to store food and laboratory chemicals, and to carry out chemical reactions. It is, however, brittle and shatters into many pieces when it strikes (or is struck by) a hard surface. Various techniques have therefore been devised to strengthen glass (see Specialty glasses below).
The main constituent of glass is silica (SiO2). Pure silica glass (also called fused quartz) does not absorb ultraviolet (UV) light and is used for applications that require transparency in this region. Ordinary glass, on the other hand, absorbs (and blocks) UV radiation. It partially blocks UVA light (wavelengths between 380 and 315 nanometers), and it totally blocks UVB (315–280 nm) and UVC (below 280 nm) radiation. This property of ordinary glass is due to the presence of compounds such as soda ash (see Ingredients in glass below).
The stability of glass also means that it does not break down in the environment. Discarded glassware needs to be properly recycled in order to avoid the buildup of trash.
Is glass a liquid?
One common misconception is that glass is a «super-cooled liquid of practically infinite viscosity» when at room temperature. Supporting evidence often cited is that many old windows appear thicker at the bottom than at the top. It is assumed that the glass was once uniform and, over time, flowed to its new shape.
It needs to be understood, however, that glass panes made in the past were usually not of uniform thickness, as noted above (see Hand-blown glass-making). When installed in a window frame, the glass would be placed thicker side down for the sake of stability and visual effects. Occasionally, such glass has been found thinner side down, probably as a result of carelessness at the time of installation.
If medieval glass flowed perceptibly, then ancient Roman and Egyptian objects should have flowed proportionately more—but this is not observed. Also, if glass flows at a rate that allows changes to be seen with the naked eye after centuries, then changes in optical telescope mirrors should be observable (by the technique of interferometry) in a matter of days—but this, too, is not observed.
In the case of refracting telescopes with large objective lenses, the lens (which is supported only around its edge) has been found to sag under its own weight. The result is a loss of focus. It occurs not because the glass flows over time but because it is not infinitely rigid. This effect, among others, limits the size of a refracting telescope. As noted by physicist Edgar Zanotto, glass at room temperature is very strongly on the solid side of the spectrum from solids to liquids.
Ingredients in glass
Although pure silica can be converted into glass for special applications, it has a melting point of about 2,000° C (3,600° F). The melting point is lowered to about 1,000°C (1,800° F) by adding either soda (sodium carbonate) or potash (potassium carbonate). The presence of soda, however, also makes the glass water soluble, which is usually undesirable. Therefore, lime (calcium oxide) is added to the mix, to restore insolubility. The resultant glass contains about 70 percent silica and is called soda-lime glass. This type of glass accounts for about 90 percent of manufactured glass.
Besides soda and lime, other ingredients are usually added to impart a variety of properties to glass. For instance, when lead oxide is added, the resultant «lead crystal» has a much higher refractive index than normal glass, and consequently much greater sparkle. The addition of barium also increases the refractive index. If boron is added, it alters the thermal properties of glass, making it more resistant to breakage during rapid changes in temperature. This is a feature of Pyrex glass, a brand name for borosilicate glass.
Thorium oxide was formerly used in producing high-quality lenses, but it is radioactive and has been replaced by lanthanum oxide. Large amounts of iron are used in glass that absorbs infrared energy, such as for heat-absorbing filters in movie projectors. Cerium(IV) oxide can be used for glass that absorbs ultraviolet (UV) radiation, which damages DNA and living tissue. Additional ingredients may be added to produce glass in a wide range of colors (see below).
Biologically active ingredients
Scientists can now make glass vessels in which biologically active molecules, such as enzymes, are embedded. These vessels can then be used to carry out reactions catalyzed by the enzymes. Preparation of this type of glass, however, cannot be carried out by the usual, high-temperature methods, because the heat would destroy the enzymes. Instead, it is prepared by an innovative approach that falls in the category known as polymerization. One example of this approach is called the sol-gel process.
Ingredients for colors
Metallic additives in the glass mix can produce a variety of colors. Here cobalt has been added to produce a bluish colored decorative glass.
The inside of a blue glass cup.
Glass with a wide range of colors can be produced by adding different metals and metal oxides during the manufacturing process. For instance, when manganese is added in small amounts, it removes the green tint lent by iron; and at higher concentrations, it produces an amethyst color. Selenium, likewise, can be used at low concentrations to decolorize glass, but at higher concentrations, it imparts a reddish color. At low concentrations (0.025–0.1 percent), cobalt yields blue glass.
The oxides of tin, antimony, and arsenic produce an opaque white glass, first used in Venice to produce an imitation porcelain. Copper oxide, at a concentration of 2–3 percent, produces a turquoise color.
Metallic gold at low concentrations (around 0.001 percent) produces a rich, ruby-colored glass, and at lower concentrations it produces a less intense red, often marketed as «cranberry.» Pure, metallic copper produces a dark red, opaque glass, and it is sometimes used as a substitute for gold in the production of ruby-colored glass. Nickel, depending on its concentration, produces blue, violet, or even black glass. Adding titanium produces yellowish-brown glass.
Uranium (0.1–2 percent) can be added to give glass a fluorescent yellow or green color. Uranium glass is typically not radioactive enough to be dangerous, but if ground into a powder (such as by scraping with sandpaper) and inhaled, it can be carcinogenic. Silver compounds (notably silver nitrate) can produce a range of colors, from orange-red to yellow. The way the glass is heated and cooled can significantly affect the colors produced by these compounds, but the chemistry involved is complex and not well understood. New shades of colored glass are frequently discovered.
Uses of glass
Glass is an extremely useful material, serving both practical and artistic needs. Many objects in homes and offices are made of glass. Drinking glasses, bowls, and bottles are often made of glass, as are light bulbs and mirrors. The picture tubes of computer monitors and televisions are also made from this material. Retail stores use glass cases to display as well as protect their products for sale.
Scientific research laboratories are equipped with flasks, test tubes, beakers, thermometers, measuring devices, and other pieces of apparatus made from glass. For these applications, borosilicate glass (such as Pyrex) is usually used for its strength and resistance to thermal shock. Most large laboratories need so much custom glassware that they keep a glassblower on staff.
Glass is also valuable for making lenses, with surfaces that are convex, concave, planar, or a combination of these types. Depending on its shape, a lens can concentrate or diverge light. Lenses are useful for such things as magnifying glasses, eyeglasses, cameras, microscopes, binoculars, and telescopes.
For certain applications, quartz glass (made of pure silica) is used, although it is more difficult to produce. Pure silica glass is also used to make fiber optic cables that can transmit light over long distances and are therefore useful for the telecommunications industry. Undersea cables have sections doped with erbium, which amplify transmitted signals by laser emission from within the glass itself.
Glass has been used in buildings since the eleventh century. It is typically used as a transparent material for windows and other architectural features. It may also be used for internal, glazed partitions. Glass in buildings can be of a safety type, including wired, toughened, and laminated glasses. Glass fiber insulation is common in roofs and walls. Foamed glass, made from waste glass, can be used as lightweight insulation.
The artistic uses of glass are discussed under Glass art.
Specialty glasses
Laminated glass
Automobile windshield displaying a «spiderweb» cracking pattern typical of laminated safety glass.
Laminated glass, invented in 1903 by French chemist Edouard Benedictus, is a type of safety glass that holds together when shattered. It is currently produced by bonding two or more layers of ordinary, annealed glass with a plastic interlayer, usually polyvinyl butyral (PVB). A typical laminated makeup would be 3 mm glass / 0.38 mm interlayer / 3 mm glass, referred to as «6.38 laminated glass.» In the event of breakage, the interlayer keeps the glass layers bonded and prevent them from falling apart into sharp pieces. This produces a characteristic «spiderweb» cracking pattern when the impact is not enough to completely pierce the glass.
Laminated glass is normally used when there is a possibility of human impact, or if the glass could fall if shattered. Shop front glazing and automobile windshields are typically laminated glasses. The PVB interlayer also gives the glass a much higher sound insulation rating, due to its damping effect, and blocks 99% of transmitted UV light.
Multiple laminates and thicker glass increases the strength. Bulletproof glass panels, made up of thick glass (often toughened) and several interlayers, can be as thick as 50 mm. A similar glass is often used for the front windows of airliners.
Toughened glass
A vandalized phone booth with toughened glass.
Toughened glass (or «tempered glass») is a type of safety glass that is typically four to six times the strength of annealed glass. That strength, however, comes with a penalty. Due to the balancing of stresses in the glass, any damage to the edges will cause the material to shatter into thumbnail sized pieces. For this reason, the glass must be cut to size before toughening and cannot be reworked once toughened. Polishing the edges or drilling holes in the glass is carried out before the toughening process starts. Also, ironically, the toughened glass surface is not as hard as annealed glass and is more susceptible to scratching.
Toughened glass is made from annealed glass via a thermal tempering process. The glass is placed on a roller table, taking it through a furnace that heats it to above its annealing temperature (600 °C). The glass is then rapidly cooled with forced drafts of air, causing the surface to harden and contract, while the inner portion of the glass remains soft for a short time. As the inner layer contracts, it induces stresses in the surface, giving the material increased strength.
Chemically strengthened glass
Chemically strengthened glass is another type of glass with increased strength. It is prepared by immersing the glass in a potassium nitrate bath at 450º C. This causes sodium ions in the glass surface to be replaced by potassium ions from the bath solution. The potassium ions, being larger than sodium ions, «wedge» into the gaps left by the sodium ions. The glass surface goes into a state of compression, while the core is in compensating tension. As a result, the glass is strengthened.
Unlike toughened glass, chemically strengthened glass has little or no bow or warp, optical distortion, or strain pattern. When broken, chemically strengthened glass still shatters in long, pointed splinters, similar to float glass. For this reason, it is not considered a safety glass and must be laminated if needed as a safety glass.
Glass art
Glass sculpture by Dale Chihuly at an exhibition in Kew Gardens, London, England. The piece is 13 feet (4 meters) high.
Glass art includes the creation of stained glass, working glass in a torch flame (lampworking), glass beadmaking, glass casting, glass fusing, and creating artistic shapes through glass blowing. Dating back to prehistoric times, glass art was extensively developed in Egypt and Assyria, brought to the fore by the Romans, and attained some of its greatest triumphs in the stained glass rose-windows created for European cathedrals.
Decorative items made of glass include vases, bowls, chandeliers, sculptures, paperweights, beads, and marbles. Often the glass is colored during the production process, but sometimes it is painted. The term «crystal glass» (derived from rock crystal) is currently applied to high-quality, colorless glass, often containing lead, but it is occasionally used in reference to any fine, hand-blown glass.
There are many techniques for creating fine glass art; each is suitable for certain kinds of objects and not for others. One important technique is called lampworking, in which the glass is melted with a flame and reshaped. In earlier years, lampworkers used oil-fueled lamps, but the modern practice is to use a torch fueled with propane or natural gas. Another technique is to manipulate glass in a kiln. Traditional stained glass work is commonly called cold glasswork. Glass can also be cut with a diamond saw and polished to give gleaming facets.
Some famous artists in glass include Lino Tagliapietra, Rene Lalique, Dale Chihuly, and Louis Comfort Tiffany. Important contemporary artists who have produced painted glass objects include Judith Schaechter and Walter Lieberman. Well-known lampworkers include Roger Parramore and Bandhu Scott Dunham. The Harvard Museum of Natural History has a collection of extremely detailed models of flowers lampworked by Leopold Blaschka and his son Rudolph [1].
Great ateliers like Tiffany, Lalique, Daum, Galle, the Corning schools in upper New York State, and Stubbe glassworks have taken glass art to its highest levels. In addition, Murano, a small group of islands north of Venice, has been home to master glassmakers for many centuries.
Stained glass
Stained glass is an art form with a long history. Many churches have beautiful stained-glass windows. The term stained glass generally refers to glass that has been colored by adding metallic salts during its manufacture. For example, copper can be used to produce green or blue glass. The molten glass is then annealed in a furnace to produce sheets of colored glass.
Early artists working with stained glass were limited to a few primary colors, but today almost any color can be produced. If fine details such as shadows or outlines are required, the artist paints them on the cold glass with special paint made from metal oxides. The piece is then fired in a kiln, causing the oxides to fuse permanently with the glass. Stained glass is now available in a variety of textures that alter the color transmission characteristics of the glass and produce surprising effects.
The process of making stained-glass windows involves cutting colored glass into different shapes, assembling the pieces (using lead came strips or copper foil), soldering them together, and installing them in a frame. This process requires artistic skill to conceive of the design and engineering skill to assemble the structure so that it can support its own weight and (for a window) survive the elements.
History of stained glass
Begun in Eastern Asia and among Muslim designers, the art of stained glass reached its height in the Middle Ages, particularly 1150-1250. As the solid Romanesque wall was eliminated, the use of glass dramatically expanded. Integrated with the lofty verticals of Gothic cathedrals, large windows afforded greater illumination that was regarded as symbolic of divine grace.
In the nineteenth century, Romanticism and the Gothic revival caused renewed interest in stained glass. Important contributions to the art were made by William Morris (English, 1834-1898), Edward Burne-Jones (English, 1833-1898), John La Farge (American, 1835-1910) and Louis Comfort Tiffany (American, 1848-1933).
After centuries of repetition and little innovation, stained glass underwent a major renaissance of form. The impetus for this new modern glass was the restoration of thousands of church windows throughout Europe, destroyed by World War II. German artists led the way, as Ervin Bossanyi, Ludwig Schaffrath, Johannes Shreiter, and many others transformed an ancient art form into a contemporary one.
References
ISBN links support NWE through referral fees
- «Do Cathedral Glasses Flow?» Am. J. Phys., 66 (May 1998), pp 392–396
- Noel C. Stokes; The Glass and Glazing Handbook; Standards Australia; SAA HB125-1998
- Glass Beads from Anglo-Saxon Graves: A Study on the Provenance and Chronology of Glass Beads from Anglo-Saxon Graves, Based on Visual Examination by Birte Brugmann
External links
All links retrieved June 23, 2017.
- Corning Museum of Glass, especially Research, Teach, and Learn section.
- Is glass liquid or solid? by Philip Gibbs
- Does Glass Flow?
- The Stained Glass Museum (Ely, England)
- Stained Glass Photography
A glass ball with coloured glass shapes inside
Edo-Kiriko, a traditional cut glass craft in Asakusa, Tokyo, Japan. The glass has two layers, a coloured layer outside a clear layer.
Glass is a hard material that can be made in many shapes. It is usually transparent, but it can also be made in colours. Glass is mainly made of silica; glass made of silica only is called silica glass.
Glass used to make windows and bottles is a specific type called soda-lime glass, composed of about 75% silicon dioxide (SiO2), sodium oxide (Na2O) from sodium carbonate (Na2CO3), calcium oxide, also called lime (CaO), and several minor additives.
By changing the proportions, and adding different ingredients, many kinds of glass can be made. Coloured glass is made by adding small amounts of metal oxides. For example, a blue colour is given by tiny amounts of cobalt oxide.
Crystal glass is made by adding lead and zinc oxides. It is not actually a crystal because all glass is a non-crystalline solid. Crystal glass is called cut glass if it has been cut by hand:
- » ‘Cut glass’ is glass that has been decorated entirely by hand by use of rotating wheels. Cuts are made in an otherwise completely smooth surface of the glass by workers holding and moving the piece against various sized metal or stone wheels».[1]
Because glass is used to make lenses, the word «glasses» often means eyeglasses.
The myth that glass is actually a liquid comes from the fact that old windows in houses and churches (200–300 years old) are sometimes a little out of shape: thicker at the bottom than the top. This is actually due to the process of glass making in the past which led to the glass pane being thicker at one edge than the other. It was sensible to install the windows with the thick edge at the bottom. Sometimes a window can be found with the thick edge at the top of the window.
Glass can be recycled over and over. Glass bottles and jars can easily be recycled to make new glass bottles and jars or used in industry as aggregate (building material) or sand.
-
Roman glass beaker from the 4th century A.D.
-
-
References
- ↑ American Cut Glass Association. [1]
Other websites
- Corning Museum of Glass
- A comprehensive guide to art glass and crystal around the world
- Working Description Furnace & Moleria — Murano Glass
- Informative website about the glass industry
- Substances used in the Making of Colored Glass Archived 2005-11-30 at the Wayback Machine
- Almost 400 articles and images about glass (mostly art glass)
Educalingo cookies are used to personalize ads and get web traffic statistics. We also share information about the use of the site with our social media, advertising and analytics partners.
Download the app
educalingo

Your glass will not do you half so much service as a serious reflection on your own minds.
Mary Astell
ETYMOLOGY OF THE WORD GLASS
Old English glæs; related to Old Norse gler, Old High German glas, Middle High German glast brightness.
Etymology is the study of the origin of words and their changes in structure and significance.
PRONUNCIATION OF GLASS
GRAMMATICAL CATEGORY OF GLASS
Glass is a verb and can also act as a noun.
A noun is a type of word the meaning of which determines reality. Nouns provide the names for all things: people, objects, sensations, feelings, etc.
The verb is the part of the sentence that is conjugated and expresses action and state of being.
See the conjugation of the verb glass in English.
WHAT DOES GLASS MEAN IN ENGLISH?
Glass
Glass is an amorphous solid material that exhibits a glass transition, which is the reversible transition in amorphous materials from a hard and relatively brittle state into a molten or rubber-like state. Glasses are typically brittle and can be optically transparent. The most familiar type of glass is soda-lime glass, which is composed of about 75% silicon dioxide, sodium oxide from sodium carbonate, lime, and several minor additives. The term glass is often used to refer only to this specific material. Silicate glass generally has the property of being transparent. Because of this, it has a great many applications. One of its primary uses is as a building material, traditionally as small panes set into window openings in walls, but in the 20th-century often as the major cladding material of many large buildings. Because glass can be formed or moulded into any shape, and also because it is a sterile product, it has been traditionally used for vessels: bowls, vases, bottles, jars and glasses. In its most solid forms it has also been used for paperweights, marbles, and beads.
Definition of glass in the English dictionary
The first definition of glass in the dictionary is a hard brittle transparent or translucent noncrystalline solid, consisting of metal silicates or similar compounds. It is made from a fused mixture of oxides, such as lime, silicon dioxide, etc, and is used for making windows, mirrors, bottles, etc. Other definition of glass is any compound that has solidified from a molten state into a noncrystalline form. Glass is also something made of glass, esp a drinking vessel, a barometer, or a mirror.
CONJUGATION OF THE VERB TO GLASS
PRESENT
Present
I glass
you glass
he/she/it glasses
we glass
you glass
they glass
Present continuous
I am glassing
you are glassing
he/she/it is glassing
we are glassing
you are glassing
they are glassing
Present perfect
I have glassed
you have glassed
he/she/it has glassed
we have glassed
you have glassed
they have glassed
Present perfect continuous
I have been glassing
you have been glassing
he/she/it has been glassing
we have been glassing
you have been glassing
they have been glassing
Present tense is used to refer to circumstances that exist at the present time or over a period that includes the present time. The present perfect refers to past events, although it can be considered to denote primarily the resulting present situation rather than the events themselves.
PAST
Past
I glassed
you glassed
he/she/it glassed
we glassed
you glassed
they glassed
Past continuous
I was glassing
you were glassing
he/she/it was glassing
we were glassing
you were glassing
they were glassing
Past perfect
I had glassed
you had glassed
he/she/it had glassed
we had glassed
you had glassed
they had glassed
Past perfect continuous
I had been glassing
you had been glassing
he/she/it had been glassing
we had been glassing
you had been glassing
they had been glassing
Past tense forms express circumstances existing at some time in the past,
FUTURE
Future
I will glass
you will glass
he/she/it will glass
we will glass
you will glass
they will glass
Future continuous
I will be glassing
you will be glassing
he/she/it will be glassing
we will be glassing
you will be glassing
they will be glassing
Future perfect
I will have glassed
you will have glassed
he/she/it will have glassed
we will have glassed
you will have glassed
they will have glassed
Future perfect continuous
I will have been glassing
you will have been glassing
he/she/it will have been glassing
we will have been glassing
you will have been glassing
they will have been glassing
The future is used to express circumstances that will occur at a later time.
CONDITIONAL
Conditional
I would glass
you would glass
he/she/it would glass
we would glass
you would glass
they would glass
Conditional continuous
I would be glassing
you would be glassing
he/she/it would be glassing
we would be glassing
you would be glassing
they would be glassing
Conditional perfect
I would have glass
you would have glass
he/she/it would have glass
we would have glass
you would have glass
they would have glass
Conditional perfect continuous
I would have been glassing
you would have been glassing
he/she/it would have been glassing
we would have been glassing
you would have been glassing
they would have been glassing
Conditional or «future-in-the-past» tense refers to hypothetical or possible actions.
IMPERATIVE
Imperative
you glass
we let´s glass
you glass
The imperative is used to form commands or requests.
NONFINITE VERB FORMS
Present Participle
glassing
Infinitive shows the action beyond temporal perspective. The present participle or gerund shows the action during the session. The past participle shows the action after completion.
WORDS THAT RHYME WITH GLASS
Synonyms and antonyms of glass in the English dictionary of synonyms
Translation of «glass» into 25 languages

TRANSLATION OF GLASS
Find out the translation of glass to 25 languages with our English multilingual translator.
The translations of glass from English to other languages presented in this section have been obtained through automatic statistical translation; where the essential translation unit is the word «glass» in English.
Translator English — Chinese
玻璃
1,325 millions of speakers
Translator English — Spanish
vidrio
570 millions of speakers
English
glass
510 millions of speakers
Translator English — Hindi
कांच
380 millions of speakers
Translator English — Arabic
زُجَاج
280 millions of speakers
Translator English — Russian
стекло
278 millions of speakers
Translator English — Portuguese
vidro
270 millions of speakers
Translator English — Bengali
কাচ
260 millions of speakers
Translator English — French
verre
220 millions of speakers
Translator English — Malay
Kaca
190 millions of speakers
Translator English — German
Glas
180 millions of speakers
Translator English — Japanese
ガラス
130 millions of speakers
Translator English — Korean
유리
85 millions of speakers
Translator English — Javanese
Kaca
85 millions of speakers
Translator English — Vietnamese
thủy tinh
80 millions of speakers
Translator English — Tamil
கண்ணாடி
75 millions of speakers
Translator English — Marathi
काच
75 millions of speakers
Translator English — Turkish
bardak
70 millions of speakers
Translator English — Italian
vetro
65 millions of speakers
Translator English — Polish
szkło
50 millions of speakers
Translator English — Ukrainian
скло
40 millions of speakers
Translator English — Romanian
pahar
30 millions of speakers
Translator English — Greek
γυαλί
15 millions of speakers
Translator English — Afrikaans
glas
14 millions of speakers
Translator English — Swedish
glas
10 millions of speakers
Translator English — Norwegian
glass
5 millions of speakers
Trends of use of glass
TENDENCIES OF USE OF THE TERM «GLASS»
The term «glass» is very widely used and occupies the 1.949 position in our list of most widely used terms in the English dictionary.

FREQUENCY
Very widely used
The map shown above gives the frequency of use of the term «glass» in the different countries.
Principal search tendencies and common uses of glass
List of principal searches undertaken by users to access our English online dictionary and most widely used expressions with the word «glass».
FREQUENCY OF USE OF THE TERM «GLASS» OVER TIME
The graph expresses the annual evolution of the frequency of use of the word «glass» during the past 500 years. Its implementation is based on analysing how often the term «glass» appears in digitalised printed sources in English between the year 1500 and the present day.
Examples of use in the English literature, quotes and news about glass
10 QUOTES WITH «GLASS»
Famous quotes and sentences with the word glass.
As Indian women, we are always balancing work, life, home, etc. It’s important to know that while juggling rubber balls and glass balls, the former may bounce back when you miss, but the glass balls will crack if you let them fall. So prioritise, prioritise, prioritise.
I’ve often said that all poetry is political. This is because real poems deal with a human response to reality and politics is part of reality, history in the making. Even if a poet writes about sitting in a glass house drinking tea, it reflects politics.
My memory of my mom is a wine glass in one hand and a cigarette in the other. She was a runway fashion model, and she was quite a glamorous woman.
Your glass will not do you half so much service as a serious reflection on your own minds.
I like to look at the glass half full.
For watching sports, I tend to drink Guinness; early evenings always begin well with a Grey Goose and tonic with plenty of lime; and on a cold winter’s night, there’s nothing quite like a glass of Black Maple Hill… an absolute peach of a bourbon.
In my new IFC comedy game show, ‘Bunk,’ we actually use our intern Patrick as a human timer — giving contestants the time it takes for him to wade through a bag of broken glass for a razor blade, to get gum out of his hair, to pick up every strand from a box of spaghetti I spill on the floor, etc, etc.
A poor self-image is the magnifying glass that can transform a trivial mistake or an imperfection into an overwhelming symbol of personal defeat.
Don’t tell me the moon is shining; show me the glint of light on broken glass.
Life is shining a light through a magnifying glass on me, looking for me to stumble. I think that’s my biggest fear.
10 ENGLISH BOOKS RELATING TO «GLASS»
Discover the use of glass in the following bibliographical selection. Books relating to glass and brief extracts from same to provide context of its use in English literature.
In a powerful sequel to Crank, Kristina is determined to manage her addiction to crack in order to keep her newborn child, but when she is unable to manage her use of the drug and the pull becomes too strong, her greatest fears are quickly …
When Rob, the charismatic leader of the senior class, turns the school nerd into Prince Charming, his actions lead to unexpected violence.
This is a startling memoir of a successful journalist’s journey from the deserted and dusty mining towns of the American Southwest, to an antique filled apartment on Park Avenue.
The role of glass in shaping the world’s history, art, and scientific achievement is made plain in this fascinating study of a ubiquitous yet absolutely essential manmade substance. (History)
Alan Macfarlane, Gerry Martin, 2002
5
Glass: Mechanics and Technology
This is the first book to discuss the correlation between the performance of industrial processes and practice-relevant properties, such as strength and optical properties.
Knowing that their father, Valentine, will soon attack, Clary and Jace must venture to the city of glass to find the warlock that has the power to save their mother’s life and then uncover the truth about their past in order to prepare for …
Starting with the dawn of vessel glassmaking in the 2nd millenium BC and ending with the work of contemporary artists, Glass covers history, social uses, design, and techniques such as blowing and engraving.
Reino Liefkes, Victoria and Albert Museum, 1997
8
Engraving Glass: A Beginner’s Guide
Step-by-step instructions for diamond burr engraving. Four complete projects plus expert advice on choosing glass, lighting and arranging finished pieces, much more. 117 illustrations. List of Supply Sources.
9
40 Great Stained Glass Projects:
«A nice collection of small stained-glass projects that would make fine gifts.
10
1000 Glass Beads: Innovation & Imagination in Contemporary …
Illustrations of magnificent bead works including jewellery and sculptural pieces.
Valerie Van Arsdale Shrader, 2004
10 NEWS ITEMS WHICH INCLUDE THE TERM «GLASS»
Find out what the national and international press are talking about and how the term glass is used in the context of the following news items.
Stained Glass Artist Puts The Pieces Together
Cynthia Anderson has been a stained glass artist in Newfield since 1974. Last month her works were displayed at the Newfield library. «ithaca.com, Jul 15»
Police: Dallas man stabbed 2 people with broken glass bottle at …
Quevedo then reportedly broke a glass bottle and began stabbing the victim in the stomach. A witness who tried to break up the fight suffered … «Dallas Morning News, Jul 15»
iPads, Google Glass helping save lives at Nemours
Abby Legates of Millsboro appeared to be in perfect health when her mother put her down for a nap one Sunday last January. But when she … «The News Journal, Jul 15»
Glass found at Kamigamojinja shrine likely came from ancient Persia
The chemical composition of a glass fragment unearthed 50 years ago at Kamigamojinja shrine in Kyoto, a World Heritage site, is a near match … «Asahi Shimbun, Jul 15»
Google Glass to make a comeback in the enterprise market
The Google Glass is not dead. Recent reports by 9to5Google indicate that Google is bringing back its smart pair of glasses, but it won’t be … «New York Daily News, Jul 15»
Glasses Malone’s Glass House 2 Is The Most Gangsta Album Cover …
Just like he told Lupe Fiasco, Glasses Malone isn’t claiming sh*t but the game and he’s proven it with his unapologetic album cover for his … «Hip-Hop Wired, Jul 15»
Let’s raise a glass to iced tea
As refreshing as a tall glass of ice tea might be, not all teas are created equal. Martha Teichner joins us for tea: Did you know that 85 percent of … «CBS News, Jul 15»
Ira Glass Negotiates Full Ownership of Famed NPR Show ‘This …
After twenty years on the air, Ira Glass has negotiated full ownership of his long-running PBS radio show, “This American Life” and its podcast … «Breitbart News, Jul 15»
Female Pilots Struggle to Break Through Glass Ceiling at Asia’s …
Female Pilots Struggle to Break Through Glass Ceiling at Asia’s Airlines … was on Southeast Asia’s female pilots and cracking the glass ceiling. «Skift, Jul 15»
Appalachian Glass offers mind-blowing array of treasures
WESTON — After working for years at Weston’s Louie Glass Co., Chip Turner decided to begin his own custom glass business 15 years ago. «The Exponent Telegram, Jul 15»
REFERENCE
« EDUCALINGO. Glass [online]. Available <https://educalingo.com/en/dic-en/glass>. Apr 2023 ».
Download the educalingo app


Discover all that is hidden in the words on
Now you see it, now you don’t. Glass is
a bit of a riddle. It’s hard enough to protect us, but it shatters with
incredible ease. It’s made from opaque sand, yet it’s completely
transparent. And, perhaps most surprisingly of all, it behaves like a
solid material… but it’s also a sort of weird liquid in disguise!
You can find glass wherever you look: most rooms in your home will
have a glass window and, if not that, perhaps a glass mirror… or a
glass lightbulb. Glass is one of the world’s oldest and most
versatile human-created materials. Let’s find out some more about it.
Photo: Glass riddle: How does something transparent to light appear colored? The colors in this glass aren’t really there! Glass lenses refract (bend) light rays of different wavelengths by different amounts, causing spectral colors to appear. This is a closeup of a Fresnel lens from a lighthouse.
Contents
- What is glass?
- How is glass made?
- Is glass a solid… or a liquid?
- What do we use glass for?
- Find out more
What is glass?
Believe it or not, glass is made from liquid sand. You can
make glass by heating ordinary sand (which is mostly made of silicon
dioxide) until it melts and turns into a liquid. You won’t find that
happening on your local beach: sand melts at the incredibly high
temperature of 1700°C (3090°F).
When molten sand cools, it doesn’t turn back into
the gritty yellow stuff you started out with: it undergoes a complete
transformation and gains an entirely different inner structure. But it
doesn’t matter how much you cool the sand, it never quite sets into a
solid. Instead, it becomes a kind of frozen liquid or what materials
scientists refer to as an amorphous solid.
It’s like a cross
between a solid and a liquid with some of the crystalline order of a
solid and some of the molecular randomness of a liquid.
Glass is such a popular material in our homes
because it has all kinds of really useful properties. Apart from
being transparent, it’s inexpensive to make, easy to shape when it’s
molten, reasonably resistant to heat when it’s set, chemically inert
(so a glass jar doesn’t react with the things you put inside it), and
it can be recycled any number of times.
Photo: Stained glass is made by adding salts of metals such as iron, manganese,
chromium, and tin to the ingredients of molten glass to give it a variety of attractive colors.
This stained glass window, designed by artist
Edward Burne-Jones, is in St Philip’s Cathedral, Birmingham, England.
How is glass made?
When US scientists tested a
prototype of the
atomic bomb in the New Mexico desert in 1945, the explosion turned
the sand in the immediate area of the impact into glass. Fortunately,
there are easier and less extreme ways of making glass—but all of
them need immense amounts of heat.
In a commercial glass plant, sand
is mixed with waste glass (from recycling collections), soda ash
(sodium carbonate), and limestone (calcium carbonate) and heated in a
furnace. The soda reduces the sand’s melting point, which helps to
save energy during manufacture, but it has an unfortunate drawback:
it produces a kind of glass that would dissolve in water! The
limestone is added to stop that happening. The end-product is called soda-lime-silica glass. It’s the ordinary glass we
can see all around us.
Artwork: Glassmaking simplified: mix and heat sand and recycled glass with calcium carbonate and
sodium carbonate.
Once the sand is melted, it is either poured into
molds to make bottles, glasses, and other containers, or «floated»
(poured on top of a big vat of molten tin metal) to make perfectly flat
sheets of glass for windows.
Photo: Perfectly flat panes of glass of uniform thickness are made by floating molten
glass on giant tanks filled with molten tin. After cooling and solidifying, the glass is cut to whatever size is needed.
Unusual glass containers are still sometimes made
by «blowing» them. A «gob» (lump) of molten glass is wrapped
around an open pipe, which is slowly rotated. Air is blown through
the pipe’s open end, causing the glass to blow up like a balloon.
With skillful blowing and turning, all kinds of amazing shapes can be
made.
Photo: Making a piece of glass artwork by blowing and spinning. The glass (right) has been blown into a sphere on the long metal pipe you can see on the top right. It’s now being turned on the same pipe to shape it further. Note how two hot blow torches are being applied from the left to keep the glass molten and flexible so it can be worked into shape. Photo by James Bunn courtesy of US Army and
DVIDS.
Glass makers use a slightly different process
depending on the type of glass they want to make. Usually, other
chemicals are added to change the appearance or properties of the
finished glass. For example, iron and chromium-based chemicals are
added to the molten sand to make green-tinted glass. Oven-proof borosilicate glass (widely sold under the
trademark PYREX®) is
made by adding boron oxide to the molten mixture. Adding lead oxide
makes a fine crystal glass that can be cut more easily; highly prized
cut lead crystal sparkles with color as it refracts (bends) the light
passing through it. Some special types of glass are made by a
different manufacturing process. Bulletproof
glass is made from a sandwich or laminate of multiple layers of glass and plastic bonded
together. Toughened glass used in car windshields is made by cooling molten
glass very quickly to make it much harder.
Stained (colored) glass is made by adding metallic compounds to glass while it is molten; different
metals give the separate segments of glass their different colors.
Photo: Borosilicate glass, such as this PYREX® jug (back), can withstand extreme changes of temperature, unlike normal glass (front), which shatters. The ordinary glass jar at the front is quite a bit thinner and considerably lighter. You can also see,
very clearly that the borosilicate glass is a slightly blueish color (as is the boron oxide from which it’s made).
Is glass a solid… or a liquid?
Artwork: Top: In a regular crystalline solid, the atoms are arranged in
a neat and predictable way. Bottom: In an amorphous solid, such as glass, the arrangement is much more random.
It’s a very interesting question.
The answer is both—and neither! There are widely differing opinions
on how to refer to materials such as glass that seem to be a bit like
liquids in some ways and a bit like solids in others.
In schools and in books, we tend to learn that solids all have a
fixed structure of atoms.
In fact, there are different kinds of solids that have very
different structures and not everything we describe as «solid» behaves
in exactly the same way. Think of a lump of iron
and a lump of rubber. Quite clearly they are
both solids, and yet the rubber is very different from the iron.
Inside, rubber and iron have their atoms (in
the case of iron) and molecules (in the case of rubber) arranged in
totally different ways. Iron has a regular or crystalline structure
(like a climbing frame with atoms at the corners), while rubber is a polymer (made from long chains of molecules
loosely connected together). Or think of water.
As you may have discovered, water is an almost unique solid because it
expands to begin with when it freezes. In short, not everything fits
neatly into our ideas of solid, liquid, and gas and not all solids,
liquids, and gases behave in a nice, neat, easy-to-explain way. The
exceptions are the things that make science really interesting!
Amorphous solids
Let’s return to glass. Peer through a microscope
inside some glass and you’ll find the molecules from which it’s made
are arranged in an irregular pattern. That’s why glass is sometimes
referred to as an amorphous solid (a solid without the regular
crystalline structure that something like a metal would have). You may
also see glass described as a «frozen supercooled liquid». This is
another way of saying «glass is a liquid that has never set», which is
the puzzling statement you’ll sometimes find in science books. We could
say glass is a bit like a liquid and a bit like a solid. It has an
internal structure that is somewhere between the structure of a liquid
and a solid, with some of the order of a solid and some of the
randomness of a liquid.
Glass is by no means the only amorphous solid. It’s possible to make
a type of water called amorphous ice that could be described as
in-between solid (water) and liquid (ice). You do this by cooling water
very quickly. The ice forms so fast that it doesn’t have time to build
up its normal, crystalline structure. So what you get looks like ice
but behaves in some ways like liquid water. Other substances can be
made into amorphous solids too. Solar cells are often made from
something called amorphous silicon.
Photo: A solar panel made from amorphous silicon.
Photo by Dennis Schroeder courtesy of
NREL (US Department of Energy/National Renewable
Energy Laboratory) (photo id #22143).
What do we use glass for?
Photo: Glass can be used to recycle other
materials. Uranium glass has an unusual yellow-green color and glows in
ultraviolet light.
These glass pieces were made using waste uranium from the cleanup of
the Fernald uranium processing plant near Cincinnati, Ohio, USA.
Vitrification (turning a material into glass) is one way to dispose of
nuclear waste safely.
Picture by courtesy of US Department of Energy.
Glass starts your day with a sparkle: a glance at your watch, a gaze through the glaze
at the sun or the rain, a frown in the mirror, a song from the
shower, as you wash with water trickling down warm from the
solar panels
on the roof. Glasses pack the breakfast table, which might, itself,
be made from smoked glass, and there are bottles and jars of all
shapes and colors. Making breakfast in your kitchen, you might be
using a glass-ceramic cooktop or a
microwave with a metal-lined
window to keep the waves inside. Maybe you’re watching croissants warm
through the Pyrex oven door? (And is that a glass teapot?)
When you check your email over breakfast (bad habit), speed-of-light Internet data zips
to your home through optical fibers, just as sunlight streams
through the heat-reflective windows that keep you cool. You read the
words through the glass LCD panel of your laptop or the toughened
gorilla glass of your smartphone, both charged by solar energy
from photovoltaic panels on the roof. Talking heads are
muttering at you through the TV screen in the corner.
Then you set off for work or school, in a glass-wrapped car, bus, train (perhaps even
helicopter), hunched under low-energy lamps covered by glass to make them last. If you’re driving, the highway you’re
roaring down could be made from aggregates and asphalt including
recycled glass; even the white stripes down the middle use tiny glass
beads to make them shine in your headlights. Maybe you drop in the
bank or the post office on your way, smiling at the cashier behind
her bulletproof window, as you make a quick copy of
your driving licence (which you carelessly leave behind on the glass plate
of the photocopier).
Photo: Glass brings the outside in! This is the wonderful Wayfarers Chapel in Rancho Palos Verdes, California, designed by Lloyd Wright (son of Frank Lloyd Wright). Picture from The Jon B. Lovelace Collection of California Photographs in Carol M. Highsmith’s America Project, Library of Congress, Prints and Photographs Division.
If it’s a modern building, your office or school might be a mini glass cathedral; we
think of glass as brittle and fragile, but toughen it the right way
and you can make walls, floors, roofs, and staircases from it; shops
show their wares through huge, laminated panels, polished to
perfection.
And that’s only a tiny selection of the things glass does for us. There are
loads more places you’ll find it hiding, from the bulbs in
thermometers
and the cermet fillings in teeth to the fiberglass hulls of boats,
the «sandpaper» we use for decorating (which is often glasspaper), and
even the strain gauges that warn us when buildings are cracking. Clear,
clean, attractive, unreactive, cheap, strong, and effective. What
more could you want? Glass is one of those magic materials we absolutely take for granted;
everywhere and nowhere—»invisibly transparent,» so
we don’t even notice that it’s there!
Find out more
On this website
- Bulletproof glass
- Electrochromic (electrically darkening) glass
- Heat-reflecting (low-E) glass
- Materials science
- Photochromic (light-sensitive) glass
On other sites
- Museum of Glass: A large glass museum in Tacoma, Washington.
- The Corning Museum of Glass: Explore the history of glass and glassmaking in Corning, New York.
- The National Glass Centre: A glass museum at the University of Sunderland, UK.
Books
- The Glass Bathyscaphe: How Glass Changed the World
by Alan MacFarlane and Gerry Martin by Alan Macfarlane and Gerry Martin. Profile, 2002. Explores the history of glass from ancient to modern times. I believe this is the same book (differently packaged) as Glass: A World History by Alan Macfarlane and Gerry Martin. University of Chicago Press, 2002. - Introduction to Glass Science and Technology by J.E. Shelby. Royal Society of Chemistry, 2020. An undergraduate text covering the chemical and materials-science aspects of glass. Covers the various different types of glass and their mechanical, optical, and other properties.
- Glass: Mechanics and Technology by Eric Le Bourhis. Wiley-VCH, 2014. Covers the history, structure, properties, and applications of glass.
- Glass Science by Robert Doremus. Wiley, 1994. A classic single-volume guide to the science of amorphous, glassy solids.
- Atoms Under the Floorboards by Chris Woodford. Bloomsbury, 2015. If you’re looking for a more light-hearted approach, my recent book explores the wonders of glass in «Chapter 8: Amazing Glazing.» You might be able to read some it online on Google Books by following this link.
Articles
- For the Sake of Art: Risk and Reward at 2,000 Degrees by Gloria Dawson. The New York Times, September 1, 2016. This slideshow goes behind the scenes at UrbanGlass, an experimental glass workshop in New York City.
- Glass works: how Corning created the ultrathin, ultrastrong material of the future by Bryan Gardiner, Wired, September 24, 2012. The origins of a remarkable glass-ceramic material that eventually became smartphone Gorilla Glass.
- Blow by Blow: GlassLab Comes to Governors Island by Julia Felsenthal. The New York Times, July 3, 2012. Introducing GlassLab at the Corning Museum of Glass.
- Willow Glass: ultra-thin glass can ‘wrap’ around devices by Katia Moskvitch, BBC News, June 5, 2012. Corning reveals a thin and flexible glass for next-generation displays.
- The Glass Whisperer by Andrea Truppin. The New York Times, January 27, 2005. The world of Michael Davis, a specialist in restoring antique glass.
- Glass Blowing Becomes an Art Form by Katherine Pearson. The New York Times, January 7, 1979. From the Times archive, a short introduction to the wonders of blown glass.
Podcasts
- BBC Radio 4: In Our Time: The Science of Glass by Melvyn Bragg, May 28, 2015. Interviewed guests include physicist Professor Athene Donald. (43-minutes, can be downloaded as an MP3.)
Patents
For deeper technical detail, try these:
- US Patent 1,304,623: Glass by Eugene C. Sullivan and William C. Taylor, Corning, May 27, 1919. One of Corning’s original Pyrex (borosilicate glass) patents, which describes its chemical composition and physical properties.
- US Patent 1,304,623: Sodium aluminosilicate glass article strengthened by a surface compressive stress layer by David Boyd, Corning, December 11, 1973. Corning’s patent for the super-strong «Gorilla glass» that Apple used to such great effect in its smartphones and tablets.
- US Patent 20160368777: Water solvated glass/amorphous solid ionic conductors by John B. Goodenough et al, December 22, 2016. One of the 20th-century’s most innovative chemists proposes a completely new kind of battery based on glass.


- Entertainment & Pop Culture
- Geography & Travel
- Health & Medicine
- Lifestyles & Social Issues
- Literature
- Philosophy & Religion
- Politics, Law & Government
- Science
- Sports & Recreation
- Technology
- Visual Arts
- World History
- On This Day in History
- Quizzes
- Podcasts
- Dictionary
- Biographies
- Summaries
- Top Questions
- Infographics
- Demystified
- Lists
- #WTFact
- Companions
- Image Galleries
- Spotlight
- The Forum
- One Good Fact
- Entertainment & Pop Culture
- Geography & Travel
- Health & Medicine
- Lifestyles & Social Issues
- Literature
- Philosophy & Religion
- Politics, Law & Government
- Science
- Sports & Recreation
- Technology
- Visual Arts
- World History
- Britannica Explains
In these videos, Britannica explains a variety of topics and answers frequently asked questions. - Britannica Classics
Check out these retro videos from Encyclopedia Britannica’s archives. - Demystified Videos
In Demystified, Britannica has all the answers to your burning questions. - #WTFact Videos
In #WTFact Britannica shares some of the most bizarre facts we can find. - This Time in History
In these videos, find out what happened this month (or any month!) in history.
- Student Portal
Britannica is the ultimate student resource for key school subjects like history, government, literature, and more. - COVID-19 Portal
While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today. - 100 Women
Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians. - Saving Earth
Britannica Presents Earth’s To-Do List for the 21st Century. Learn about the major environmental problems facing our planet and what can be done about them! - SpaceNext50
Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!