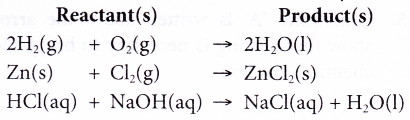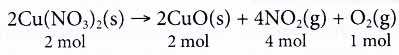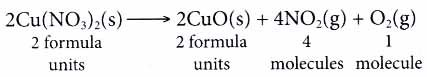In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
There are some key words to watch for when reading or writing a word equation. The words «and» or «plus» mean one chemical and another are both reactants or products. The phrase «is reacted with» indicates the chemicals are reactants. If you say «forms», «makes», or «yields», it means the following substances are products.
When you write a chemical equation from a word equation, the reactants always go on the lefthand side of the equation, while the reactants are on the righthand side. This is true even if the products are listed before the reactants in the word equation.
Key Takeaways: Word Equations
- A word equation is an expression of a chemical reaction or mathematical equation using words rather than letters, numbers, and operators.
- In chemistry, a word equation indicates the order of events of a chemical reaction. The number of moles and types of reactants yield the number of moles and types of products.
- Word equations help in learning chemistry because they reinforce the thought process involved in writing a chemical reaction or equation.
Word Equation Examples
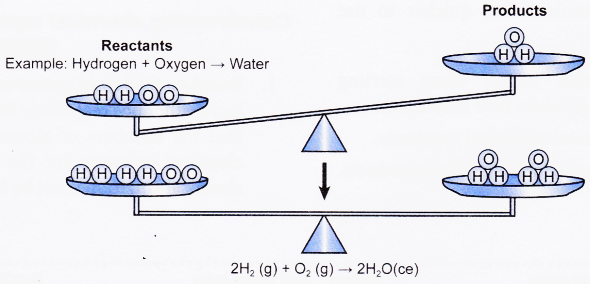
The chemical reaction 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as:
hydrogen gas + oxygen gas → steam
As a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen.»
While a word equation doesn’t ordinarily include numbers or symbols (Example: You wouldn’t say «Two H two and one O two makes two H two O», sometimes it is necessary to use a number to indicate the oxidation state of a reactant so that a person writing a chemical equation can do it correctly. This is mostly for the transition metals, which can have multiple oxidation states.
For example, in the reaction between copper and oxygen to form copper oxide, the chemical formula of copper oxide and the number of copper and oxygen atoms involved depends on whether copper(I) or copper(II) participates in the reaction. In this case, it would be fine to say:
copper + oxygen → copper(II) oxide
or
Copper reacts with oxygen to produce copper two oxide.
The (unbalanced) chemical equation for the reaction would start out as:
Cu + O2 → CuO
Balancing the the equation yields:
2Cu + O2 → 2CuO
You would get a different equation and product formula using copper(I):
Cu + O2 → Cu2O
4Cu + O2 → 2Cu2O
More examples of word reactions include:
- Chlorine gas reacts with methane and carbon tetrachloride to produce hydrogen chloride.
- Adding sodium oxide to water produces sodium hydroxide.
- Iodine crystals and chlorine gas react to make solid iron and carbon dioxide gas.
- Zinc and lead two nitrate make zinc nitrate and lead metal.
which means: Zn + Pb (NO3)2 → Zn(NO3)2 + Pb
Why Use Word Equations?
When you’re learning general chemistry, work equations are used to help introduce the concepts of reactants, products, the direction of reactions, and to help you understand precision of language. They may seem annoying, but are a good introduction to the thought processes required for chemistry courses. In any chemical reaction, you need to be able to identify the chemical species that react with each other and what they make.
Word Equations in Other Sciences
Chemistry isn’t the only science to use equations. Physics equations and mathematical equations may also be expressed in words. Usually in these equations two statements are set to be equal to each other. For example, if you way «force equals mass multiplied by acceleration» then you are providing the word equation for the formula F = m*a. Other times, one side of the equation may be less than (<), greater than (>), less than or equal to, or greater than or equal to the other side of the equation. Addition, subtraction, multiplication, division, logs, square roots, integrals, and other operations can be stated in word equations. However, complex equations that contain parentheses to describe the order of operations are very hard to understand as word equations.
Source
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (December 14, 2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
Learning outcomes
At the end of this, you should be able to:
– State how you know a reaction has taken place
-Write a word equation for a given reaction
– Write a balanced symbol equation for a reaction, given the symbols for the reactants
– Write a balanced symbol equation for a reaction when you have to look up the symbols from a table and/or using the Periodic table (Higher only)
What you should already know
– What an element is– What a compound is– The difference between ionic and covalent compounds– What a molecule is– How to write the formula for a molecule or compound using symbols
Word equations and balanced symbol equations
A reaction may happen when two or more chemicals are mixed. Sometimes you have to add heat (energy) before the reaction will occur.
Not all chemicals react together when they are mixed. We can tell a reaction has taken place if we see bubbles of gas, a change in colour, a change in temperature or a precipitate (a layer of solid in the bottom of the test tube that wasn’t there when you started). So vinegar (an acid) reacts with sodium bicarbonate – there are bubbles of gas and the test tube gets warm – but it doesn’t react with table salt (sodium chloride). The salt dissolves but nothing else happens, even if you heat the mixture.
A word equation helps us work out what has happened during a reaction.
Let’s look at the word equation for the reaction between sulphuric acid and calcium carbonate (limestone or marble).
We know a reaction happens because we see bubbles of gas and the test tube gets warm.
The clue to what the gas is is in the name of the reactants; if you add acid to a carbonate you always get carbon dioxide. (You could also test the gas; we know it is carbon dioxide because if we bubble it through limewater, the limewater goes ‘milky’.) In addition, if you react an acid with an alkali or base, like a carbonate, an oxide or a hydroxide, you always get water. (You need to know this for the chemistry exam.) The name of the acid gives you a clue to the name of the salt it forms.
Calcium carbonate + sulphuric acid→ calcium sulfate + carbon dioxide + water
Let’s look at another reaction. This is one you learn about in biology; respiration.
The reactants are glucose and oxygen, the products are carbon dioxide and water.
Glucose + oxygen→ carbon dioxide + water (+energy)
(This is almost the same as burning a hydrocarbon; you get the same products.)
Some reactions are very hard to describe in any other way than by a word equation. Not all substances have a chemical formula you can write down, for example, wood or a chocolate biscuit. Or the chemical formula may be one you don’t know, such as vinegar which is mainly a solution of something called ethanoic acid.
Word equations describe what is happening, but it is often more useful to know what each of the atoms, molecules or ions is doing. To do this, we use a balanced symbol equation. Sometimes you will have to look up the formulas for the compounds or even work them out using the Periodic Table.
‘Balancing’ just means making sure you have the same number of each sort of atom on each side.
This is important. We do not lose or gain mass during a chemical reaction. This is called ‘Conservation of mass’. You may have come across this idea already in Physics.
Let’s see how to write a balanced formula equation for the reaction between magnesium and hydrochloric acid. You know a reaction happens because there is fizzing. If you test the gas with a lighted splint, you get a ‘squeaky pop’, telling you hydrogen has been produced.
The word equation is
magnesium + hydrochloric acid → hydrogen + magnesium chloride.
If you look in the table of ions, you will see that magnesium makes ++ ions and chloride ions are – , so you need 2 chlorine ions to each ion of magnesium. You could also work this out by looking at the Periodic Table. Magnesium is in the second column of the Periodic table and chlorine is in the next-to-last column, so magnesium has 2 electrons in its outer shell to give away, and chlorine has 7 electrons in its outer shell, so needs 1 to make a full shell.
To write the symbol equation, first write down the reactants and products in symbol form: (mathrm{Mg}+mathrm{HCl} rightarrow mathrm{MgCI}_{2}+mathrm{H}_{2})
This doesn’t balance; we have 1 H and 1 Cl on the left-hand side and two of each on the right-hand side. So we need to add another HCl to make the equation balance:
(mathrm{Mg}+2 mathrm{HCl} rightarrow mathrm{MgCI}_{2}+mathrm{H}_{2})
The hydrochloric acid is in solution, and so is the magnesium chloride. You can show this by writing a little ‘aq’ (short for aqueous, which means ‘in water’) under the HCl and (mathrm{MgCI}_{2}). You could also show that the magnesium chloride is ionic and in solution by writing (mathrm{Mg}++(mathrm{Cl}-)_{2}) instead of MgCl.
You have probably seen the reaction between a group I metal and water; for example sodium and water. The sodium races around fizzing and there is a yellow flame too.
The fizzing tells us there is a gas being produced. The flame tells us there is also a lot of heat being produced; enough to ignite the gas. There is definitely a reaction going on!
The only substances involved are sodium and water and if you test the water afterwards with pH or litmus paper, you will find it is alkaline. The gas gives a ‘squeaky pop’ if it is tested with a lighted splint; it must be hydrogen.
Sodium + water→ hydrogen + sodium hydroxide.
In symbols:
(mathrm{Na}+mathrm{H}_{2} mathrm{O} rightarrow mathrm{H}_{2}+mathrm{NaOH})
This doesn’t balance, there are too many H’s on the right-hand side. If we add another Na, we also need another (mathrm{H}_{2} mathrm{O}) to make sure we have enough OH.
So we have:
(2 mathrm{Na}+2 mathrm{H}_{2} mathrm{O} rightarrow 2 mathrm{Na} mathrm{OH}+mathrm{H}_{2})
This is an example of a very important reaction; make sure you learn it, and the one for Group II metals. You’ll find that in the ‘some to try for yourself’ section.
Another reaction you might have seen or done is the reaction between copper II oxide and dilute sulphuric acid. This is a reaction requiring heat (and a lot of stirring) to make it happen. Copper II oxide (CuO) is a black insoluble powder. Dilute sulphuric is a clear liquid. The products are copper II sulfate and water. Copper II sulfate is a pretty blue colour. You can tell this reaction has happened because you have a change in colour.
Copper II oxide + dilute sulphuric acid → copper sulfate + water
Write the symbols:
(mathrm{Cu} mathrm{O}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{CuSO}_{4}+mathrm{H}_{2} mathrm{O})
There is the same number of atoms on each side; the equation is balanced. The dilute sulphuric acid and copper sulfate are both in solution, so you could write ‘aq’ underneath or show them as ions.
Let’s look at something which needs more balancing: the reaction between iron and chlorine. These are both elements and you aren’t adding anything else, so the product will be iron III chlorides, as long as you have lots of chlorine. Chlorine makes Cl- ions and iron III ions are +++.
Chlorine always comes as Cl2 molecules.
Iron + chlorine → iron III chloride
Write a formula equation:
(mathrm{Fe}+mathrm{CI}_{2} rightarrow mathrm{FeCI}_{3})
This doesn’t balance: you have 2 chlorines on the left-hand side and 3 on the right-hand side. You need to use a bit of clever maths here; 2 x 3 = 6. If you put a 3 in front of the (mathrm{CI}_{2}) and a 2 in front of the (mathrm{FeCI}_{3}), you have 6 chlorines on both sides. However, you now also have to put a 2 in front of the Fe on the left-hand side because you have 2 irons on the right-hand side:
(2 mathrm{Fe}+3 mathrm{CI}_{2} rightarrow 2 mathrm{FeCI}_{3})
You will find this ‘balancing trick’ with 2 and 3 useful for a lot of iron and also aluminium compounds. (The only — ion you are likely to come across is phosphate.) There is one to try in the ‘some to try for yourself’ section. But first here’s another example.
Let’s look at the reaction between aluminium hydroxide and sulphuric acid.It’s a reaction between an alkali and an acid, so we will get water as well as salt.
The salt is aluminium sulfate, and we can use the balancing trick to work out it is (mathrm{AI}_{2}left(mathrm{SO}_{4}right)_{3}).
Aluminium hydroxide + sulphuric acid → aluminium sulfate + water.
Begin by writing down the symbols:
(mathrm{Al}(mathrm{OH})_{3}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right)_{3}+mathrm{H}_{2} mathrm{O})
We can see straight away that we need 2 aluminium ions and 3 molecules of sulphuric acid on the left-hand side to make the aluminium sulfate.
(2 mathrm{Al}(mathrm{OH})_{3}+3 mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right) 3+mathrm{H}_{2} mathrm{O})
Now we have 6 OH’s and 6 H’s on the left. Each O needs 2 H’s to make water, so we have the right amount to make 6 (mathrm{H}_{2} mathrm{O}) with nothing left over.
The final balanced equation is:
(2 mathrm{Al}(mathrm{OH})_{3}+3 mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{Al}_{2}left(mathrm{SO}_{4}right)_{3}+6 mathrm{H}_{2} mathrm{O})
Take your time and remember to make sure you have the same number of each element or ion on each side.
You might find it helps to write the symbols in groups and cross them off as you use them:
These symbol equations all involve elements and ions, but we can write balanced symbol equations for other reactions, too.
If we burn the hydrocarbon gas propane in oxygen (or in the air), we get carbon dioxide and water.
Propane + oxygen → carbon dioxide + water
The formula for propane is (mathrm{C}_{3} mathrm{H}_{8}).
Write down a symbol equation:
(mathrm{C}_{3} mathrm{H}_{8}+mathrm{O}_{2} rightarrow mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
You can see straight away that there are 3C’s on the left and 1 on the right, so you know there must be 3 (mathrm{CO}_{2}) ‘s. The 8 H’s will make 4 water molecules. So you need 4O’s for the water and 6 for the (mathrm{CO}_{2}) ‘s; 10 O’s or (5 mathrm{O}_{2}) ‘s.
The balanced equation is:
(mathrm{C}_{3} mathrm{H}_{8}+5 mathrm{O}_{2} rightarrow 3 mathrm{CO}_{2}+4 mathrm{H}_{2} mathrm{O})
Sometimes you end up with an odd number of O’s or H’s. If this happens, you just double everything up.
Propane’s cousin ethane C2H6 needs this:
(2 mathrm{C}_{2} mathrm{H}_{6}+7 mathrm{O}_{2} rightarrow 4 mathrm{CO}_{2}+6 mathrm{H}_{2} mathrm{O})
Summary
- You know a reaction has happened because you get bubbles of gas, a change in colour, a change in temperature or a precipitate.
- You can write a reaction as a word equation. This is useful if you don’t know the formula or for complicated compounds/substances.
- You can also write a reaction as a balanced symbol equation. Balanced means having the same number of atoms of each element on each side of the equation.
- You might need to use the Periodic Table or a table of ions to work out the formulas of the things you are reacting.
- You need to know what happens when you react an acid with an oxide, a hydroxide or a carbonate.
- You need to know what happens when you react to a Group I or II metal with water.
- You need to know what happens when you burn a hydrocarbon.
- You need to be able to write word equations for respiration and photosynthesis.
Some to try for yourself
- Write a word equation for the reaction that occurs when a candle is burned. (Candle wax is a hydrocarbon.)
- Write a word equation for the reaction between potassium and water.
- Write a balanced formula equation for the reaction between calcium metal and water.
- Write a balanced formula equation for the reaction between magnesium carbonate (left(mathrm{MgCO}_{3}right)) and sulphuric acid (left(mathrm{H}_{2} mathrm{SO}_{4}right)).
- Write a balanced formula equation for the reaction between iron II oxide (FeO) and nitric acid (left(mathrm{HNO}_{3}right)).
- Write a balanced formula equation for the reaction between sodium carbonate and hydrochloric acid.
- Ammonia (left(mathrm{NH}_{3}right)) is made by reacting together nitrogen and hydrogen.
- Write a balanced formula equation for this reaction.
- Iron is extracted from haematite iron ore (Iron III oxide) by heating with carbon. Write a balanced formula equation for this reaction. Write a balanced formula equation for the reaction between aluminium oxide and nitric acid.
- Quicklime is made by heating limestone, calcium carbonate. A gas is given off during the reaction. Quicklime is alkaline and reacts with water to form calcium hydroxide. Write balanced formula equations for these two reactions?
Answers
Remember to write a word equation before you write the balanced formula equation to make sure you don’t forget anything.
Answer 1
candle wax + oxygen → carbon dioxide + water
Answer 2
potassium + water → potassium hydroxide + hydrogen
Answer 3
calcium + water → calcium hydroxide + hydrogen
(mathrm{Ca}+2 mathrm{H}_{2} mathrm{O} rightarrow mathrm{Ca}(mathrm{OH})_{2}+mathrm{H}_{2})
Calcium is Group II so it is ++. Otherwise, it’s the same as the reaction between sodium and water. The calcium fizzes and the test tube gets warm.
Answer 4
magnesium carbonate + sulphuric acid → magnesium sulfate + carbon dioxide + water
(mathrm{MgCO}_{3}+mathrm{H}_{2} mathrm{SO}_{4} rightarrow mathrm{MgSO}_{4}+mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
In the acid/carbonate reaction, the carbonate ion gives an oxygen atom to the hydrogen from the acid to form a water molecule.
Magnesium carbonate is the chemical name for gypsum, a soft white rock.
Answer 5
Iron II oxide + nitric acid → iron II nitrate + water
(mathrm{FeO}+2 mathrm{HNO}_{3} rightarrow mathrm{Fe}left(mathrm{NO}_{3}right)_{2}+mathrm{H}_{2} mathrm{O})
Transition metals like iron and copper often have more than one ‘oxidation state’ – they can have different numbers of +. Watch out for things like iron II and copper I.
Answer 6
Sodium carbonate + hydrochloric acid → sodium chloride + carbon dioxide + water
(mathrm{Na}_{2} mathrm{CO}_{3}+2 mathrm{HCl} rightarrow 2 mathrm{NaCl}+mathrm{CO}_{2}+mathrm{H}_{2} mathrm{O})
Something alkaline + an acid gives a salt. This reaction gives ‘table salt’. Sodium carbonate is washing soda.
Answer 7
(mathrm{N}_{2}+3 mathrm{H}_{2} rightarrow 2 mathrm{NH}_{3})
This is an important reaction because it is used to make ammonia for fertiliser.It is what is called an ‘equilibrium’ reaction; not all the reactants are turned into a product. You will learn more about this reaction in a later unit of your course.
Answer 8
Iron III oxide + carbon → iron + carbon dioxide
(2 mathrm{Fe}_{2} mathrm{O}_{3}+3 mathrm{C} rightarrow 2 mathrm{Fe}+3 mathrm{CO}_{2})
You will learn about the extraction of metals later in your course.
Answer 9
Aluminium oxide + nitric acid → aluminium nitrate + water
(mathrm{Al}_{2} mathrm{O}_{3}+6 mathrm{HNO}_{3} rightarrow 2 mathrm{Al}left(mathrm{NO}_{3}right)_{3}+3 mathrm{H}_{2} mathrm{O})
Aluminium reacts in a similar way to group II metals but forms +++ ions.
Since there are 2 Al ions there need to be (6 mathrm{NO}_{3}) ions.
Answer 10
The gas given off is carbon dioxide. This means that the first reaction is
(mathrm{CaCO}_{3} rightarrow mathrm{CO}_{2}+mathrm{CaO})
This tells you that quicklime is calcium oxide.
The calcium oxide is reacted with water. Nothing is given off, so the product must be calcium hydroxide. This is known as slaked lime.
(mathrm{CaO}+mathrm{H}_{2} mathrm{O} rightarrow mathrm{Ca}(mathrm{OH})_{2})
These are very important reactions.
Calcium oxide is used in the manufacture of cement, concrete and mortar (used to stick bricks together). A lot of heat is given out by the second reaction (it is exothermic) – if you’ve ever mixed cement you will know it gets hot. This is why.
Lime is also used to ‘sweeten’ clay soils to make them easier to work and more productive.
Slaked lime is dissolved in water to make lime water, which you use to test for carbon dioxide. The milkiness is due to the particles of insoluble calcium carbonate formed when calcium hydroxide reacts with carbon dioxide.
(mathrm{Ca}(mathrm{OH})_{2}+mathrm{CO}_{2} rightarrow mathrm{CaCO}_{3}+mathrm{H}_{2} mathrm{O})
All chemical reactions are represented by chemical equations. A chemical equation is a shorthand representation of a chemical reaction using the symbols and formulae of substance involved in the chemical reaction.
The symbols and formulae of the substances (elements or compounds) are arranged to show the reactants and products of a chemical reaction.
A chemical reaction occurs when starting substances react to produce new substances.
(a) Starting substances are called reactants.
(b) New substances formed are called products.
In an equation, the reactants are written at the left-hand side whereas the products are written at the right-hand side.
For example:
People also ask
- What is the Relative Atomic Mass and Relative Molecular Mass of an Element?
- What is One Mole and How many Particles are in a Mole?
- How do you Calculate the Molar Mass of a Substance?
- What is the Molar Volume of a Gas at STP?
- How do you know the Order of Elements in a Chemical Formula
- What is Empirical and Molecular Formula?
Constructing chemical equations
1. Based on the law of conservation of mass, matter can neither be created nor destroyed. This means that the numbers of atoms before and after a chemical reaction are the same. Therefore, a chemical equation must be balanced.
2. Table below shows how a chemical equation can be constructed.
Table: Constructing a chemical equation.
| Reaction: Iron filings react with copper(II) chloride to produce iron(III) chloride solution and copper. | |
| Step | Explanation and example |
| Identify the reactants, products and their formulae. | Reactants: Iron, Fe and copper(II) chloride, CuCl2 Products : Iron(III) chloride, FeCl3 and copper, Cu |
| Write the main part of the equation. | Fe + CuCl2 ⟶ FeCl3 + Cu Reactants Products |
| Determine the number of atoms of each element on both sides of the equation. | Left-hand side Right-hand side Fe atom : 1 Fe atom : 1 Cu atom : 1 Cu atom : 1 Cl atom : 2 Cl atom : 3 The numbers of atoms are not balanced. |
| Balance the equation by adjusting the coefficients. (Note: Coefficients are the numbers in front of the formulae.) |
The Cl atoms are balanced. Fe + 3CuCl2 ⟶ 2FeCl3 + Cu As a result, the numbers of Fe atoms and Cu atoms are not balanced. The Fe atoms are then balanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + Cu Lastly, the Cu atoms are balanced. 2Fe + 3CuCI2 ⟶ 2FeCI3 + 3Cu |
| Check that the equation is balanced. | Left-hand side Right-hand side Fe atom : 2 Fe atom : 2 Cu atom: 3 Cu atom : 3 Cl atom : 6 Cl atom : 6 Now, the equation is balanced. |
| Put in the state symbol of each substance. | 2Fe(s) + 3CuCl2(aq) ⟶ 2FeCl3(aq) + 3Cu(s) |
3. The state symbols (s), (l) and (g) represent the solid, liquid and gaseous states respectively. The symbol (aq) represents an aqueous solution.
4. Sometimes the symbol ‘↑’ is used to indicate the release of a gas.
5. Sometimes ‘△’ is written above the arrow to show that heating is necessary to bring about a chemical reaction.
Rules for writing chemical equation:
Certain rules have to be followed while writing a chemical equation.
- The reactants taking part in the reaction are written in terms of their symbols or molecular formulae on the left-hand side of the equation.
- A plus (+) sign is added between the formulae of the reactants.
- The products of reaction are written in terms of their symbols or molecular formulae on the right-hand side of the equation.
- A plus (+) sign is added between the formulae of the products.
- In between the reactants and the products an arrow sign (⟶) is inserted to show which way the reaction is occurring.
A + B ⟶ C + D
In this chemical equation, A and B are the reactants, and C and D are the products. The arrow indicates that the reaction proceeds towards the formation of C and D.
How to Balance Chemical Equations?
The first step in balancing an equation is to count the number of atoms of each element on both sides of the equation. For example, reactants X and Y2 react to form a compound XY. The word equation for this reaction would be
X + Y2 ⟶ XY
The number of atoms of elements X and Y in the above-mentioned equation is shown below.
| Element | Number of atoms in LHS | Number of atoms in RHS |
| X | 1 | 1 |
| Y | 2 | 1 |
To balance Y on both sides, multiply RHS by 2, i.e.,
X + Y2 ⟶ 2XY
Now, the number of atoms of Y is balanced but not the number of atoms of X. Therefore, multiply X on the LHS by 2. Thus, the equation becomes
2X + Y2 ⟶ 2XY
This is a balanced equation as the number of atoms of X and Y on both sides is equal.
Keeping these steps in mind, let us now write the chemical equation for the formation of magnesium oxide.
Step 1: Magnesium burns in oxygen to give magnesium oxide. Here, the reactants are magnesium and oxygen. The product is magnesium oxide.
Step 2: Thus, the word equation is
Magnesium + Oxygen ⟶ Magnesium oxide
Step 3: Replacing the names with symbols and formulae, we get the chemical equation as
Mg + O2 ⟶ MgO
Step 4: The number of atoms of the elements are
| Element | Number of atoms in LHS | Number of atoms in RHS |
| Magnesium | 1 | 1 |
| Oxygen | 2 | 1 |
To balance oxygen on both sides, multiply RHS by 2, i.e.,
Mg + O2 ⟶ 2MgO
Now, the number of oxygen atoms is balanced but the number of magnesium atoms is not. Therefore, multiply magnesium on the LHS by 2. Thus, the equation becomes
2Mg + O2 ⟶ 2MgO
This is the balanced chemical equation.
Constructing Balanced Chemical Equations
Aim: To construct balanced chemical equations.
Materials: Copper(II) carbonate powder, limewater, concentrated hydrochloric acid, concentrated
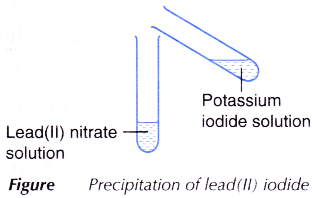
ammonia solution, lead(II) nitrate solution and potassium iodide solution.
Apparatus: Test tubes, stoppers, rubber bung with delivery tube, test tube holder, Bunsen burner and glass tube.
Procedure:
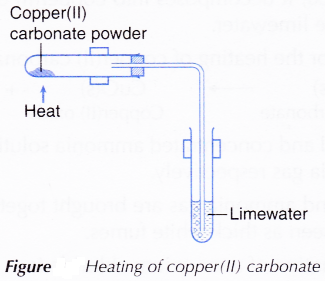
A. Heating of copper(II) carbonate
- Half a spatula of copper(II) carbonate powder is placed into a test tube.
- The apparatus is set up as shown in Figure.
Figure: Heating of copper(II) carbonate
- Copper(II) carbonate is heated and the gas produced is passed through limewater.
- The changes to copper(II) carbonate and limewater are observed.
- When the reaction is completed, the delivery tube is withdrawn from the limewater and the Bunsen burner is removed.
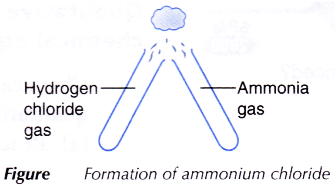
B. Formation of ammonium chloride
- Using a glass tube, three or four drops of concentrated hydrochloric acid are dropped in a test tube. The test tube is stoppered and left aside for a few minutes.
- Using a clean glass tube, step 1 is repeated using concentrated ammonia solution.
- Both stoppers are removed and the mouths of the test tubes are brought together as shown in Figure.
- All observations are recorded.
C. Precipitation of lead(II) iodide
- 2 cm3 of potassium iodide solution is added to 2 cm3 of lead(II) nitrate solution as shown in Figure.
- The mixture is shaken and any change is observed.
Observations:
| Section | Observation | Inference |
| A | Copper(II) carbonate changes colour from green to black. Limewater turns milky. |
Copper(II) carbonate decomposes into copper(II) oxide, which is black in colour. Carbon dioxide is released. |
| B | Thick white fumes are produced at the mouth of the test tubes. | The white fumes are solid ammonium chloride. |
| C | A yellow precipitate is produced. | The yellow precipitate is lead(II) iodide. |
Discussion:
- When copper(II) carbonate is heated, it decomposes into copper(II) oxide and carbon dioxide. The presence of carbon dioxide is detected by the limewater.
- Therefore, the balanced equation for the heating of copper(II) carbonate is
- The concentrated hydrochloric acid and concentrated ammonia solution are left for a few minutes to produce hydrogen chloride gas and ammonia gas respectively.
- When the hydrogen chloride gas and ammonia gas are brought together, they react to form fine white solids of ammonium chloride. These are seen as thick white fumes.
- The balanced equation for the formation of ammonium chloride is
- When the colourless lead(II) nitrate solution is added to the colourless potassium iodide solution, yellow precipitate of lead(II) iodide is produced. At the same time, colourless potassium nitrate solution is also produced.
- The balanced equation for the precipitation of lead(II) iodide is
Qualitative and quantitative aspects of chemical equations
- Chemical equations give us the following qualitative information.
(a) Reactants and products of a chemical reaction.
(b) Physical states of the reactants and products. - Take the following equation as an example.
2C(s) + O2(g) ⟶ 2CO(g)
From the equation, we know that the reactants are solid carbon and oxygen gas. The product of the reaction is carbon monoxide gas. - Quantitatively, the coefficients in a balanced equation tell us the exact proportions of reactants and products in a chemical reaction.
Take the following equation as an example.
From the equation, we know that 2 moles of copper(II) nitrate decompose into 2 moles of copper(II) oxide, 4 moles of nitrogen dioxide gas and 1 mole of oxygen gas. - At the microscopic level, the coefficients in a chemical reaction tell us the number of particles 1 involved in the reaction.
- A chemical equation serves as an important ; communicative tool for chemists.
(a) A chemical equation precisely describes a chemical reaction.
(b) Chemists use chemical equations to solve quantitatively-related problems.
Note:
- Qualititatively, hydrogen gas reacts with oxygen gas to give water.
- Qualitatively, 2 molecules (or 2 moles) of hydrogen gas react with 1 molecule (or 1 mole) of oxygen gas to give 2 molecules (or 2 moles) of water.
Chemical Reactions
Word Equations
- Write word equations that describe chemical reactions.
What’s for dinner?
Various ways of recording recipes have developed over the centuries. The cookbook shown above was written by a woman who probably collected all her own recipes. Later, printed cookbooks became available (even guys had no excuse for not being able to cook). Today we can find recipes on a number of internet sites and can quickly search for information on how to cook anything we want. Reading a recipe sometimes requires we understand a few codes and symbols (what’s the difference between a tsp and a Tsp?), but the information on what we start with and what we end up with is there.
Writing Chemical Equations
Chemical reactions are occurring all around you. Plants use sunlight to drive their photosynthetic process and produce energy. Cars and other vehicles burn gasoline in order to power their engines. Batteries use electrochemical reactions to produce energy and power many everyday devices. Many chemical reactions are going on inside you as well, especially during the digestion of food.
In math class, you have written and solved many mathematical equations. Chemists keep track of chemical reactions by writing equations as well. In any chemical reaction one or more substances, called reactants , are converted into one or more new substances, called products . The general form of the equation for such a process looks like this.
Unlike in a math equation, a chemical equation does not use an equal sign. Instead the arrow is called a yield sign and so the equation is described as “reactants yield products”.
Word Equations
You can describe a chemical reaction by writing a word equation . When silver metal is exposed to sulfur it reacts to form silver sulfide. Silver sulfide is commonly known as tarnish and turns the surface of silver objects dark and streaky black (see Figure below ). The sulfur that contributes to tarnish can come from traces of sulfur in the air or from food such as eggs. The word equation for the process is:
The silver and the sulfur are the reactants in the equation, while the silver sulfide is the product.
Figure 11.1
The coffee percolator on the left has been tarnished from exposure to sulfur. Tarnish is the chemical compound silver sulfide. The same percolator on the right has been polished with a tarnish removal product in order to restore its silver finish.
Another common chemical reaction is the burning of methane gas. Methane is the major component of natural gas and is commonly burned on a gas stove or in a Bunsen burner ( Figure below ). Burning is a chemical reaction in which some type of fuel is reacted with oxygen gas. The products of the reaction in the burning of methane as well as other fuels are carbon dioxide and water. The word equation for this reaction is:
Figure 11.2
A Bunsen burner is commonly used to heat substances in a chemistry lab. Methane is reacted with oxygen to form carbon dioxide and water.
Word equations can be very useful, but do have one major drawback. They cannot be used for any quantitative work. A word equation does not tell how many moles of each material are needed or how many moles of product are formed.
Summary
- Word equations are used to describe the conversion of reactants to products.
Practice
Questions
Read the material at the link below and do the practice problems:
Practice Problems
Review
Questions
- Write the generic form of a chemical reaction.
- What are reactants?
- What are products?
- chemical reaction : Conversion of reactants to products
- product: The result of chemical reaction
- reactant: The starting material for a chemical reaction
- word equation: A description of a chemical reaction using the names of the compounds.
Chemical Equations
- Describe the symbols used in a chemical equation.
How do you make Shrimp gumbo?
Shrimp gumbo is one of many enjoyable dishes that are part of the Cajun culture in Louisiana. It’s a spicy dish that needs careful control of all the ingredients so that it has a “kick”, but is not overwhelming. Recipes tell not only what is in the preparation, but describes how much of each ingredient and details of how to cook the meal. Similarly, We need this type of information in order to carry out chemical reactions successfully and safely.
Chemical Equations
Word equations are time-consuming to write and do not prove to be convenient for many of the things that chemists need to do with equations. A chemical equation is a representation of a chemical reaction that displays the reactants and products with chemical formulas. The chemical equation for the reaction of methane with oxygen is shown:
The equation above, called a skeleton equation, is an equation that shows only the formulas of the reactants and products with nothing to indicate the relative amounts. The first step in writing an accurate chemical equation is to write the skeleton equation, making sure that the formulas of all substances involved are written correctly. All reactants are written to the left of the yield arrow, separated from one another by a plus sign. Likewise, products are written to the right of the yield arrow, also separated with a plus sign.
It is often important to know the physical states of the reactants and products taking part in a reaction. To do this, put the appropriate symbol in parentheses after each formula: ( s ) for solid, ( l ) for liquid, ( g ) for gas, and ( aq ) for an aqueous (water-based) solution. The previous reaction becomes:
The Table below shows a listing of symbols used in chemical equations. Some, such as the double arrow which represents equilibrium, and the use of a catalyst in a reaction, will be treated in detail in other concepts.
| Symbol | Description |
| + | Used to separate multiple reactants or products |
| yield sign; separates reactants from products | |
| replaces the yield sign for reversible reactions that reach equilibrium | |
| ( s ) | reactant or product in the solid state |
| ( l ) | reactant or product in the liquid state |
| ( g ) | reactant or product in the gas state |
| ( aq ) | reactant or product in an aqueous solution (dissolved in water) |
| formula written above the arrow is used as a catalyst in the reaction | |
| triangle indicates that the reaction is being heated |
Summary
Click on the image above for more content
Review
Questions
- What does a skeleton equation tell you?
- Why would you want to know the physical state of materials?
- What does the symbol
mean?
- If I see
over the arrow, what will I do?
- chemical equation: A representation of a chemical reaction that displays the reactants and products with chemical formulas
- skeleton equation: An equation that shows only the formulas of the reactants and products with nothing to indicate the relative amounts.
Balancing Equations
- Balance chemical equations when given the skeleton information.
Any Leftovers?
When you cook a meal, quite often there are leftovers because you prepared more than people would eat at one sitting. Sometimes when you repair a piece of equipment, you end up with what are called “pocket parts”, small pieces you put in your pocket because you’re not sure where they belong. Chemistry tries to avoid leftovers and pocket parts. In normal chemical processes, we cannot create or destroy matter (law of conservation of mass). If we start out with ten carbon atoms, we need to end up with ten carbon atoms. John Dalton’ atomic theory said that chemical reactions basically involve the rearrangement of atoms. Chemical equations need to follow these principles in order to be correct.
Balancing Chemical Equations
A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can write a chemical equation for the reaction of carbon with hydrogen gas to form methane (CH 4 ).
In order to write a correct equation, you must first write the correct skeleton equation with the correct chemical formulas. Recall that hydrogen is a diatomic molecule and so is written as H 2 .
When we count the number of atoms of both elements, shown under the equation, we see that the equation is not balanced. There are only 2 atoms of hydrogen on the reactant side of the equation, while there are 4 atoms of hydrogen on the product side. We can balance the above equation by adding a coefficient of 2 in front of the formula for hydrogen.
A coefficient is a small whole number placed in front of a formula in an equation in order to balance it. The 2 in front of the H 2 means that there are a total of atoms of hydrogen as reactants. Visually, the reaction looks like the Figure below .
Figure 11.3
Reaction between carbon and hydrogen to form methane.
In the balanced equation, there is one atom of carbon and four atoms of hydrogen on both sides of the arrow. Below are guidelines for writing and balancing chemical equations.
- Determine the correct chemical formulas for each reactant and product.
- Write the skeleton equation.
- Count the number of atoms of each element that appears as a reactant and as a product. If a polyatomic ion is unchanged on both sides of the equation, count it as a unit.
- Balance each element on at a time by placing coefficients in front of the formulas. No coefficient is written for a 1. It is best to begin by balancing elements that only appear in one formula on each side of the equation. NEVER change the subscripts in a chemical formula – you can only balance equations by using coefficients.
- Check each atom or polyatomic ion to be sure that they are equal on both sides of the equation.
- Make sure that all coefficients are in the lowest possible ratio. If necessary, reduce to the lowest ratio.
Sample Problem: Balancing Chemical Equations
Aqueous solutions of lead(II) nitrate and sodium chloride are mixed. The products of the reaction are an aqueous solution of sodium nitrate and a solid precipitate of lead(II) chloride.
Step 1: Plan the problem.
Follow the steps for writing and balancing a chemical equation.
Step 2: Solve.
Write the skeleton equation with the correct formulas.
Count the number of each atom or polyatomic ion on both sides of the equation.
| reactants | products |
| 1 Pb atom | 1 Pb atom |
| 2 NO 3 – ions | 1 NO 3 – ions |
| 1 Na atom | 1 Na atom |
| 1 Cl atom | 2 Cl atoms |
The nitrate ions and the chlorine atoms are unbalanced. Start by placing a 2 in front of the NaCl. This increases the reactant counts to 2 Na atoms and 2 Cl atoms. Then place a 2 in front of the NaNO 3 . The result is:
The new count for each atom and polyatomic ion becomes:
| reactants | products |
| 1 Pb atom | 1 Pb atom |
| 2 NO 3 – ions | 2 NO 3 – ions |
| 2 Na atom | 2 Na atom |
| 2 Cl atoms | 2 Cl atoms |
Step 3: Think about your result.
The equation is now balanced since there are equal numbers of atoms of each element on both sides of the equation.
Summary
- The process of balancing chemical equations is described.
Practice
Questions
Get some experience in balancing chemical equations at the following web site:
http://www.sciencegeek.net/APchemistry/APtaters/EquationBalancing.htm
Review
Questions
- What is the law of conservation of mass?
- How did Dalton describe he profess of a chemical reaction?
- Why don’t we change the subscripts in order to balance an equation?
- balanced equation: A chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation.
- coefficient: A small whole number placed in front of a formula in an equation in order to balance it.
Combination Reactions
- Define combination reaction.
- Write products of combination reactions when given the reactants.
How useful is a wheel rim?
A wheel rim is not very useful by itself. Driving on the rim can damage it and make for a very rough ride. When the rim is combined with a tire, the product can be put on a car and used for a safe and comfortable ride. The two separate items have combined to make something that improves the car ride.
Combination Reactions
A combination reaction is a reaction in which two or more substances combine to form a single new substance. Combination reactions can also be called synthesis reactions. The general form of a combination reaction is:
One combination reaction is two elements combining to form a compound. Solid sodium metal reacts with chlorine gas to produce solid sodium chloride.
Notice that in order to write and balance the equation correctly, it is important to remember the seven elements that exist in nature as diatomic molecules (H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , and I 2 ).
One sort of combination reaction that occurs frequently is the reaction of an element with oxygen to form an oxide. Metals and nonmetals both react readily with oxygen under most conditions. Magnesium reacts rapidly and dramatically when ignited, combining with oxygen from the air to produce a fine powder of magnesium oxide.
This reaction can be seen in the following video: http://www.youtube.com/watch?v=NnFzHt6l4z8 (0:37).
Click on the image above for more content
Sulfur reacts with oxygen to form sulfur dioxide.
When nonmetals react with one another, the product is a molecular compound. Often, the nonmetal reactants can combine in different ratios and produce different products. Sulfur can also combine with oxygen to produce sulfur trioxide.
Transition metals are capable of adopting multiple positive charges within their ionic compounds. Therefore, most transition metals are capable of forming different products in a combination reaction. Iron reacts with oxygen to form both iron(II) oxide and iron(III) oxide.
Sample Problem: Combination Reactions
Potassium is a very reactive alkali metal that must be stored under oil in order to prevent it from reacting with air. Write the balanced chemical equation for the combination reaction of potassium with oxygen.
Step 1: Plan the problem
Make sure formulas of all reactants and products are correct before balancing the equation. Oxygen gas is a diatomic molecule. Potassium oxide is an ionic compound and so its formula is constructed by the crisscross method. Potassium as an ion becomes K + , while the oxide ion is O 2− .
Step 2: Solve
The skeleton (unbalanced) equation:
The equation is then easily balanced with coefficients.
Step 3: Think about your result
Formulas are correct and the resulting combination reaction is balanced.
Combination reactions can also take place when an element reacts with a compound to form a new compound composed of a larger number of atoms. Carbon monoxide reacts with oxygen to form carbon dioxide according to the equation:
Two compounds may also react to from a more complex compound. A very common example is the reactions of oxides with water. Calcium oxide reacts readily with water to produce an aqueous solution of calcium hydroxide.
Sulfur trioxide gas reacts with water to form sulfuric acid. This is an unfortunately common reaction that occurs in the atmosphere in some places where oxides of sulfur are present as pollutants. The acid formed in the reaction falls to the ground as acid rain.
Figure 11.4
Acid rain has severe consequences on both natural and man-made objects. Acid rain degrades marble statues like the one on the left (A). The trees in the forest on the right (B) have been killed by acid rain.
Summary
- Combination reactions occur when two or more substances combine to form a new substance.
Practice
Questions
Complete the reactions and balance the equations on the worksheet at the link below:
http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet2.pdf
Review
Questions
- What are combination reactions?
- Write the product of the following reaction:
- Is
a combination reaction? Explain your answer.
- combination reaction: A reaction in which two or more substances combine to form a single new substance.
Decomposition Reactions
- Define decomposition reaction.
- Write the products of decomposition reactions when given the reactant.
- Write the reactant of a decomposition reaction when given the products.
How does a decomposition reaction work?
Antoine Lavoisier is widely known as the “father of modern chemistry”. He was one of the first to study chemical reactions in detail. Lavoisier reacted mercury with oxygen to form mercuric oxide as part of his studies on the composition of the atmosphere. He was then able to show that the decomposition of mercuric oxide produced mercury and oxygen. The diagram above shows the apparatus used by Lavoisier to study the formation and decomposition of mercuric oxide.
Decomposition Reactions
A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. The general form of a decomposition reaction is:
Most decomposition reactions require an input of energy in the form of heat, light, or electricity.
Binary compounds are compounds composed of just two elements. The simplest kind of decomposition reaction is when a binary compound decomposes into its elements. Mercury(II) oxide, a red solid, decomposes when heated to produce mercury and oxygen gas.
Figure 11.5
Mercury(II) oxide is a red solid. When it is heated, it decomposes into mercury metal and oxygen gas.
A reaction is also considered to be a decomposition reaction even when one or more of the products are still compounds. A metal carbonate decomposes into a metal oxide and carbon dioxide gas. For example, calcium carbonate decomposes into calcium oxide and carbon dioxide.
Metal hydroxides decompose on heating to yield metal oxides and water. Sodium hydroxide decomposes to produce sodium oxide and water.
Some unstable acids decompose to produce nonmetal oxides and water. Carbonic acid decomposes easily at room temperature into carbon dioxide and water.
Sample Problem: Decomposition Reactions
When an electric current is passed through pure water, it decomposes into its elements. Write a balanced equation for the decomposition of water.
Step 1: Plan the problem
Water is a binary compound composed of hydrogen and oxygen. The hydrogen and oxygen gases produced in the reaction are both diatomic molecules.
Step 2: Solve
The skeleton (unbalanced) equation:
Note the abbreviation “elec” above the arrow to indicate the passage of an electric current to initiate the reaction. Balance the equation.
Step 3: Think about your result
The products are elements and the equation is balanced.
Summary
- A definition of decomposition reaction and example reactions are given.
Practice
Questions
Write the reactions (including names and balanced equations) as requested on the following web site:
http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet3.pdf
Review
Questions
- What is a decomposition reaction?
- What is usually needed for a decomposition reaction to take place?
- Are elements always the product of a decomposition reaction?
- decomposition reaction: A reaction in which a compound breaks down into two or more simpler substances.
Combustion Reaction
- Define combustion reaction.
- Write the products of combustion reactions when given the starting materials.
How do you cook the perfect marshmallow?
Roasting marshmallows over an open fire is a favorite past-time for campers, outdoor cook-outs, and just gathering around a fire in the back yard. The trick is to get the marshmallow a nice golden brown without catching it on fire. Too often we are not successful and we see the marshmallow burning on the stick – a combustion reaction taking place right in front of us.
Combustion Reactions
A combustion reaction is a reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat. Combustion reactions must involve O 2 as one reactant. The combustion of hydrogen gas produces water vapor.
Notice that this reaction also qualifies as a combination reaction.
Figure 11.6
Explosion of the Hindenberg.
The Hindenburg was a hydrogen-filled airship that suffered an accident upon its attempted landing in New Jersey in 1937. The hydrogen immediately combusted in a huge fireball, destroying the airship and killing 36 people. The chemical reaction was a simple one: hydrogen combining with oxygen to produce water.
Many combustion reactions occur with a hydrocarbon, a compound made up solely of carbon and hydrogen. The products of the combustion of hydrocarbons are carbon dioxide and water. Many hydrocarbons are used as fuel because their combustion releases very large amounts of heat energy. Propane (C 3 H 8 ) is a gaseous hydrocarbon that is commonly used as the fuel source in gas grills.
Practice Problem: Combustion Reactions
Ethanol can be used as a fuel source in an alcohol lamp. The formula for ethanol is C 2 H 5 OH. Write the balanced equation for the combustion of ethanol.
Step 1: Plan the problem
Ethanol and oxygen are the reactants. As with a hydrocarbon, the products of the combustion of an alcohol are carbon dioxide and water.
Step 2: Solve
Write the skeleton equation:
Balance the equation.
Step 3: Think about your result
Combustion reactions must have oxygen as a reactant. Note that the water that is produced is in the gas rather than the liquid state because of the high temperatures that accompany a combustion reaction.
Summary
- Combustion reaction is defined and examples are given.
Practice
Questions
Write the reactions and balance the equations for the questions on the sheet found on this web site:
http://www.sciencegeek.net/Chemistry/chempdfs/EquationsWorksheet6.pdf
Review
Questions
- What is needed for a combustion reaction to take place?
- What is formed in any combustion reaction?
- Mercury reacts with oxygen to form mercuric oxide. Is this a combustion reaction?
- What are the products of any combustion reaction involving a hydrocarbon?
- combustion reaction: A reaction in which a substance reactants with oxygen gas, releasing energy in the form of light and heat.
Single-Replacement Reactions
- Define single-replacement reaction.
- Give examples of single-displacement reactions.
Why is the silver dark?
The cup shown above provides an example of tarnish, a chemical reaction caused when silver metal reacts with hydrogen sulfide gas produced by some industrial processes or as a result of decaying animal or plant materials:
The tarnish can be removed using a number of polishes, but the process also removes a small amount of silver along with the tarnish.
Single-Replacement Reactions
A single-replacement reaction is a reaction in which one element replaces a similar element in a compound. The general form of a single-replacement (also called single-displacement) reaction is:
In this general reaction, element is a metal and replaces element
, also a metal, in the compound. When the element that is doing the replacing is a nonmetal, it must replace another nonmetal in a compound, and the general equation becomes:
is a nonmetal and replaces the nonmetal
in the compound with
.
Metal Replacement
Magnesium is a more reactive metal than copper. When a strip of magnesium metal is placed in an aqueous solution of copper(II) nitrate, it replaces the copper. The products of the reaction are aqueous magnesium nitrate and solid copper metal.
This subcategory of single-replacement reactions is called a metal replacement reaction because it is a metal that is being replaced (zinc).
Hydrogen Replacement
Many metals react easily with acids and when they do so, one of the products of the reaction is hydrogen gas. Zinc reacts with hydrochloric acid to produce aqueous zinc chloride and hydrogen ( Figure below ).
In a hydrogen replacement reaction, the hydrogen in the acid is replaced by an active metal.
Figure 11.7
Zinc metal reacts with hydrochloric acid to give off hydrogen gas in a single-displacement reaction.
Some metals are so reactive that they are capable of replacing the hydrogen in water. The products of such a reaction are the metal hydroxide and hydrogen gas. All group 1 metals undergo this type of reaction. Sodium reacts vigorously with water to produce aqueous sodium hydroxide and hydrogen (see Figure below ).
Figure 11.8
Sodium metal reacts vigorously with water, giving off hydrogen gas. A large piece of sodium will often generate so much heat that the hydrogen will ignite.
Halogen Replacement
The element chlorine reacts with an aqueous solution of sodium bromide to produce aqueous sodium chloride and elemental bromine.
The reactivity of the halogen group (group 17) decreases from top to bottom within the group. Fluorine is the most reactive halogen, while iodine is the least. Since chlorine is above bromine, it is more reactive than bromine and can replace it in a halogen replacement reaction.
Summary
- The activity series describes the relative reactivities of metals and halogens.
Practice
Questions
Read the material at the link below and do the practice problems:
http://www.chemteam.info/Equations/SingleReplacement.html
Review
Questions
- What is a metal replacement reaction?
- Will a non-metal replace a metal?
- What is the most reactive halogen?
- What products will I get if I add potassium metal to water?
- single-replacement reaction: A reaction in which one element replaces a similar element in a compound.
Activity Series
- Define activity series.
- Use the activity series to predict the outcome of reactions.
What’s the difference between the two pictures above?
We see above two metals that can be exposed to water. The picture on the left is of sodium, which gives a violent reaction when it comes in contact with water. The picture on the right is of silver, a metal so unreactive with water that it can be made into drinking vessels. Both metals have a single s electron in their outer shell, so you would predict similar reactivities. However, we have a better tool that allows us to make better prediction about what will react with what.
The Activity Series
Single-replacement reactions only occur when the element that is doing the replacing is more reactive than the element that is being replaced. Therefore, it is useful to have a list of elements in order of their relative reactivities. The activity series is a list of elements in decreasing order of their reactivity. Since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. The Table below is an activity series of most common metals and of the halogens.
| Activity of Metals | Activity of Halogens |
|
Li K React with cold water, replacing Ba hydrogen. Sr Ca Na |
F 2 Cl 2 Br 2 I 2 |
|
Mg Al React with steam, but not cold Zn water, replacing hydrogen. Cr Fe Cd |
|
|
Co Ni Do not react with water. React Sn with acids, replacing hydrogen. Pb |
|
| H 2 | |
|
Cu Hg Unreactive with water or acids. Ag Pt Au |
For a single-replacement reaction, a given element is capable of replacing an element that is below it in the activity series. This can be used to predict if a reaction will occur. Suppose that small pieces of the metal nickel were placed into two separate aqueous solutions: one of iron(III) nitrate and one of lead(II) nitrate. Looking at the activity series, we see that nickel is below iron, but above lead. Therefore, the nickel metal will be capable of replacing the lead in a reaction, but will not be capable of replacing iron.
In the descriptions that accompany the activity series of metals, a given metal is also capable of undergoing the reactions described below that section. For example, lithium will react with cold water, replacing hydrogen. It will also react with steam and with acids, since that requires a lower degree of reactivity.
Sample Problem: Single-Replacement Reactions
Use the activity series to predict if the following reactions will occur. If not, write NR. If the reaction does occur, write the products of the reaction and balance the equation.
A.
B.
Step 1: Plan the problem
For A, compare the placements of aluminum and zinc on the activity series. For B, compare the placements of silver and hydrogen.
Step 2: Solve
Since aluminum is above zinc, it is capable of replacing it and a reaction will occur. The products of the reaction will be aqueous aluminum nitrate and solid zinc. Take care to write the correct formulas for the products before balancing the equation. Aluminum adopts a 3+ charge in an ionic compound, so the formula for aluminum nitrate is Al(NO 3 ) 3 . The balanced equation is:
Since silver is below hydrogen, it is not capable of replacing hydrogen in a reaction with an acid.
Summary
- Metals and halogens are ranked according to their ability to displacement other metals or halogens below them in the series.
Practice
Questions
Take the quiz on the web site below:
http://www.sophia.org/chemical-reactions-activity-series-concept
Review
Questions
- What does the activity series tell us?
- Can a metal undergo any of the reactions listed below it in the series?
- List two metals that cobalt will displace and two that will displace it.
- activity series: A list of elements in decreasing order of their reactivity.
Double-Replacement Reactions
- Define double-replacement reaction.
- Predict products of double-replacement reactions when given the reactants.
Wanna trade?
The practice of barter (trading one thing for another) has been in existence from the beginning of time. In the illustration above, Items like chickens were bartered for newspapers. You have something I want, and I have something you want. So we trade and we each have something new. Some chemical reactions are like that. Compounds swap parts and you have new materials.
Double-Replacement Reactions
A double-replacement reaction is a reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds. The general form of a double-replacement (also called double-displacement) reaction is:
In this reaction, and
are positively-charged cations, while
and
are negatively-charged anions. Double-replacement reactions generally occur between substances in aqueous solution. In order for a reaction to occur, one of the products is usually a solid precipitate, a gas, or a molecular compound such as water.
Formation of a Precipitate
A precipitate forms in a double-replacement reaction when the cations from one of the reactants combine with the anions from the other reactant to form an insoluble ionic compound. When aqueous solutions of potassium iodide and lead(II) nitrate are mixed, the following reaction occurs.
There are very strong attractive forces that occur between Pb 2+ and I − ions and the result is a brilliant yellow precipitate (see Figure below ). The other product of the reaction, potassium nitrate, remains soluble.
Figure 11.9
Formation of lead iodide precipitate.
Formation of a Gas
Some double-replacement reactions produce a gaseous product which then bubbles out of the solution and escapes into the air. When solutions of sodium sulfide and hydrochloric acid are mixed, the products of the reaction are aqueous sodium chloride and hydrogen sulfide gas.
Formation of a Molecular Compound
Another kind of double-replacement reaction is one that produces a molecular compound as one of its products. Many examples in this category are reactions that produce water. When aqueous hydrochloric acid is reacted with aqueous sodium hydroxide, the products are aqueous sodium chloride and water.
Sample Problem: Double-Replacement Reactions
Write a complete and balanced chemical equation for the following double-replacement reactions. One product is indicated as a guide.
A. (hydrogen cyanide gas is formed)
B. (a precipitate of barium sulfate forms)
Step 1: Plan the problem
In A, the production of a gas drives the reaction. In B, the production of a precipitate drives the reaction. In both cases, use the ionic charges of both reactants to construct the correct formulas of the products.
Step 2: Solve
A. The cations of both reactants are +1 charged ions, while the anions are -1 charged ions. After exchanging partners, the balanced equation is:
B. Ammonium ion and nitrate ion are 1+ and 1− respectively, while barium and sulfate are 2+ and 2−. This must be taken into account when exchanging partners and writing the new formulas. Then, the equation is balanced.
Step 3: Think about your result
Both are double-replacement reactions. All formulas are correct and the equations are balanced. Occasionally, a reaction will produce both a gas and a molecular compound. The reaction of a sodium carbonate solution with hydrochloric acid produces aqueous sodium chloride, carbon dioxide gas, and water.
Summary
- The double-replacement reaction is described.
- Examples of the double-replacement reaction are shown.
Practice
Questions
Read the material at the web site below and do the practice problems:
http://www.chemteam.info/Equations/DoubleReplacement.html
Review
Questions
- What are the usual reactants in a double-replacement reaction?
- List the three possible types of products.
- Why would you not expect two ionic products?
- double-replacement reaction: A reaction in which the positive and negative ions of two ionic compounds exchange places to form two new compounds.
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
Word Equation Examples
The chemical reaction
2H2(g)+O2(g) ⟶ H2O(g)
would be expressed as
hydrogen gas + oxygen gas → steam
as a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen».
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
Word Equation Examples
The chemical reaction
2H2(g)+O2(g) ⟶ H2O(g)
would be expressed as
hydrogen gas + oxygen gas → steam
as a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen».
Chemical reaction:
- Physical Change: temporary & usually reversible change in which no new substance are formed
- e.g. Melting of ice or wax, magnetizing or demagnetizing of iron, preparation of solution, making of different objects from wood, soil, paper etc.
- Chemical Change: permanent & usually irreversible change in which new substances with different properties are formed
e.g. formation of curd from milk, rusting of iron, burning of candle, electrolysis of water etc.
| Physical change | Chemical change | ||
| 1 | Temporary change | 1 | Permanent Change |
| 2 | Usually a reversible change | 2 | Usually an irreversible change |
| 3 | New substance is not formed | 3 | New substance is formed |
| 4 | Only physical properties e.g. color, taste, physical properties are changed. | 4 | The physical properties, chemical properties and chemical composition are changed. |
| 5 | Change in energy is not usually seen. | 5 | Usually, energy is absorbed or evolved in it. |
| 6 | Change in mass (or amount of substance) is not seen e.g. dissolving salt in water | 6 | Change in mass (or amount of substance) is seen e.g. rusting of iron |
Chemical Reaction:
- The combination, decomposition or exchange that takes place in the molecules of matter during a chemical change
- The substance that take part in a chemical reaction are called reactants.
- The substance which are formed after a chemical reaction are called products.
- A chemical reaction can be expressed in word equation and chemical equation.
- Word Equation:
- The chemical reaction expressed in terms of full names of reactants and products is called word equation.
e.g. Hydrogen + Oxygen —–>Water (with electric sparks)
- Reactants are written at first and then an arrow (à) is written means “forms or gives” and then products are written. The two way arrows are used to show that the reaction is reversible.
- If reactants or products are two or more they are joined by the (+) sign.
- The required conditions for the chemical reaction can be written above the arrow to make the equation more informative.
e.g. Potassium Chlorate —–>Potassium chloride + Oxygen (with heat and MnO2)
Demerits of Word equation:
- It takes more space and time.
- The total number of atoms and molecules of reactants and products cannot be counted.
- The equation cannot be balanced.
- Chemical or Formula Equation:
- The chemical reaction expressed by writing symbols and molecular formulae of reactants and products is called chemical equation or formula equation.
- It is more informative, easy and practicable way to express a chemical reaction.
e.g. 2KClO3 —–>2KCl + 302 (in the presence of heat and MnO2)
- It can be further classified into two types. They are:
- Skeletal or Unbalanced Chemical equation:
- The chemical equation in which the total number of atoms of each element in reactants and products are not equal.
Demerits of Skeletal or Unbalanced Chemical equation:
- It does not follow the law of conservation of mass.
- It does not tell us about the ratio of reactant and product molecules.
- It does not give information about the total number of each element in reactants and products.
2. Balanced Chemical equation:
- The chemical equation written by balancing total number of atoms of each element in reactants and products
- It is more informative than the unbalanced chemical equation.
- It is based on the law of conservation of mass & Dalton’s atomic theory.
| Unbalanced Chemical Equation | Balanced Chemical Equation |
| Na + Cl2 —–> NaCl | 2Na + Cl2 —–> 2NaCl |
| Cl2 + KI —–> KCl + I2 | Cl2 + KI —–> 2KCl + I2 |
| N2+ H2 —–> NH3 | N2+ 3H2 —–> 2NH3 |
Balancing of Chemical Equation
- “Hit & Trial Method” is the simplest method to balance a chemical equation.
Steps:
- The chemical change is written correctly in the form of word equation.
- The word equation is written correctly in the form of formula equation using symbols and molecular formula.
- The number of atoms of each element are counted on each side of the chemical equation. They are balanced by adding suitable coefficients without changing the molecular formula of reactants and products.
e.g. i. Potassium Chlorate —–>Potassium chloride + Oxygen [Word Equation]
ii. KClO3 —–> KCl+ 302 [Formula Equation]
iii. 2KClO3 —–>2KCl + 302 [Balanced Equation]
- To make a chemical equation more informative, states of reactants and products can be expressed by following signs in brackets.
| (s) => solid | (aq.) =>aqueous(solution in water) |
| (g) or ↑ => gas | (ppt.) or ↓ =>precipitate |
| (l) => liquid | (conc.) => concentrated |
| ∆ => heat | (dil.) => dilute |
Information obtained from a balanced chemical equation:
- The symbol and molecular formulae of reactants & products.
- The total number of atoms or molecules of reactants & products.
- The ratio of molecular weight of reactant & product molecules.
- The type of chemical reaction.
Limitations of a balanced chemical equation:
- A balanced chemical equation may not provide information about:
- The physical state of reactants and products.
- Concentration of reactants
- Conditions required for the reaction e.g. heat, light, pressure, catalyst etc.
- The rate of chemical reaction
- The time taken for the reaction to complete
Reversible & Irreversible Reactions:
- Reversible Reaction: chemical reaction which occurs both in forward as well as backward direction
- A two way arrow (↔) is used to show such reaction
e.g. N2 +3H2 <—–> 2NH3
- Irreversible Reaction: chemical reaction which occurs only in one direction i.e. forward direction
- A single headed arrow (→) is used to show such reaction.
e.g. CaCO3 —–> CaO +CO2
Author: Sulaksha Purna Shrestha
- balanced chemical equation
- chemical change
- chemical equation
- physical change
- unbalanced chemical equation
- word equation
Presentation on theme: «Word Equations Word Equation: a short hand way of describing a chemical reaction.»— Presentation transcript:
1
Word Equations Word Equation: a short hand way of describing a chemical reaction
2
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water
3
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water reactants
4
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water reactantsproducts
5
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water reactantsproducts «produces»
6
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. special conditions spark hydrogen + oxygen water reactantsproducts «produces»
7
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water Reactants: the molecules put together to react
8
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water Products: the molecules produced in the reaction
9
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water -shows the direction of the reaction -read as «produces»
10
Word Equations Word Equation: a short hand way of describing a chemical reaction e.g. spark hydrogen + oxygen water Special Conditions: things needed to get the reaction to «go» -written above the arrow e.g.heat, electrolysis, spark