This article is about the scalar physical quantity. For an overview of and topical guide to energy, see Outline of energy. For other uses, see Energy (disambiguation).
| Energy | |
|---|---|

A plasma lamp, using electrical energy to create plasma, light, heat, movement and a faint sound |
|
|
Common symbols |
E |
| SI unit | joule |
|
Other units |
kW⋅h, BTU, calorie, eV, erg, foot-pound |
| In SI base units | J = kg⋅m2⋅s−2 |
| Extensive? | yes |
| Conserved? | yes |
| Dimension | M L2 T−2 |
In physics, energy (from Ancient Greek ἐνέργεια (enérgeia) ‘activity’) is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light. Energy is a conserved quantity—the law of conservation of energy states that energy can be converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J).
Common forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, and the internal energy contained within a thermodynamic system. All living organisms constantly take in and release energy.
Due to mass–energy equivalence, any object that has mass when stationary (called rest mass) also has an equivalent amount of energy whose form is called rest energy, and any additional energy (of any form) acquired by the object above that rest energy will increase the object’s total mass just as it increases its total energy.
Human civilization requires energy to function, which it gets from energy resources such as fossil fuels, nuclear fuel, or renewable energy. The Earth’s climate and ecosystems processes are driven by the energy the planet receives from the Sun (although a small amount is also contributed by geothermal energy).
Forms
The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the components of an object – and potential energy reflects the potential of an object to have motion, and generally is a function of the position of an object within a field or may be stored in the field itself.
While these two categories are sufficient to describe all forms of energy, it is often convenient to refer to particular combinations of potential and kinetic energy as its own form. For example, the sum of translational and rotational kinetic and potential energy within a system is referred to as mechanical energy, whereas nuclear energy refers to the combined potentials within an atomic nucleus from either the nuclear force or the weak force, among other examples.[1]
| Type of energy | Description |
|---|---|
| Mechanical | the sum of macroscopic translational and rotational kinetic and potential energies |
| Electric | potential energy due to or stored in electric fields |
| Magnetic | potential energy due to or stored in magnetic fields |
| Gravitational | potential energy due to or stored in gravitational fields |
| Chemical | potential energy due to chemical bonds |
| Ionization | potential energy that binds an electron to its atom or molecule |
| Nuclear | potential energy that binds nucleons to form the atomic nucleus (and nuclear reactions) |
| Chromodynamic | potential energy that binds quarks to form hadrons |
| Elastic | potential energy due to the deformation of a material (or its container) exhibiting a restorative force as it returns to its original shape |
| Mechanical wave | kinetic and potential energy in an elastic material due to a propagating oscillation of matter |
| Sound wave | kinetic and potential energy in a material due to a sound propagated wave (a particular type of mechanical wave) |
| Radiant | potential energy stored in the fields of waves propagated by electromagnetic radiation, including light |
| Rest | potential energy due to an object’s rest mass |
| Thermal | kinetic energy of the microscopic motion of particles, a kind of disordered equivalent of mechanical energy |
History
Thomas Young, the first person to use the term «energy» in the modern sense.
The word energy derives from the Ancient Greek: ἐνέργεια, romanized: energeia, lit. ‘activity, operation’,[2] which possibly appears for the first time in the work of Aristotle in the 4th century BC. In contrast to the modern definition, energeia was a qualitative philosophical concept, broad enough to include ideas such as happiness and pleasure.
In the late 17th century, Gottfried Leibniz proposed the idea of the Latin: vis viva, or living force, which defined as the product of the mass of an object and its velocity squared; he believed that total vis viva was conserved. To account for slowing due to friction, Leibniz theorized that thermal energy consisted of the motions of the constituent parts of matter, although it would be more than a century until this was generally accepted. The modern analog of this property, kinetic energy, differs from vis viva only by a factor of two. Writing in the early 18th century, Émilie du Châtelet proposed the concept of conservation of energy in the marginalia of her French language translation of Newton’s Principia Mathematica, which represented the first formulation of a conserved measurable quantity that was distinct from momentum, and which would later be called «energy».
In 1807, Thomas Young was possibly the first to use the term «energy» instead of vis viva, in its modern sense.[3] Gustave-Gaspard Coriolis described «kinetic energy» in 1829 in its modern sense, and in 1853, William Rankine coined the term «potential energy». The law of conservation of energy was also first postulated in the early 19th century, and applies to any isolated system. It was argued for some years whether heat was a physical substance, dubbed the caloric, or merely a physical quantity, such as momentum. In 1845 James Prescott Joule discovered the link between mechanical work and the generation of heat.
These developments led to the theory of conservation of energy, formalized largely by William Thomson (Lord Kelvin) as the field of thermodynamics. Thermodynamics aided the rapid development of explanations of chemical processes by Rudolf Clausius, Josiah Willard Gibbs, and Walther Nernst. It also led to a mathematical formulation of the concept of entropy by Clausius and to the introduction of laws of radiant energy by Jožef Stefan. According to Noether’s theorem, the conservation of energy is a consequence of the fact that the laws of physics do not change over time.[4] Thus, since 1918, theorists have understood that the law of conservation of energy is the direct mathematical consequence of the translational symmetry of the quantity conjugate to energy, namely time.
Units of measure
Joule’s apparatus for measuring the mechanical equivalent of heat. A descending weight attached to a string causes a paddle immersed in water to rotate.
In 1843, James Prescott Joule independently discovered the mechanical equivalent in a series of experiments. The most famous of them used the «Joule apparatus»: a descending weight, attached to a string, caused rotation of a paddle immersed in water, practically insulated from heat transfer. It showed that the gravitational potential energy lost by the weight in descending was equal to the internal energy gained by the water through friction with the paddle.
In the International System of Units (SI), the unit of energy is the joule, named after Joule. It is a derived unit. It is equal to the energy expended (or work done) in applying a force of one newton through a distance of one metre. However energy is also expressed in many other units not part of the SI, such as ergs, calories, British thermal units, kilowatt-hours and kilocalories, which require a conversion factor when expressed in SI units.
The SI unit of energy rate (energy per unit time) is the watt, which is a joule per second. Thus, one joule is one watt-second, and 3600 joules equal one watt-hour. The CGS energy unit is the erg and the imperial and US customary unit is the foot pound. Other energy units such as the electronvolt, food calorie or thermodynamic kcal (based on the temperature change of water in a heating process), and BTU are used in specific areas of science and commerce.
Scientific use
Classical mechanics
In classical mechanics, energy is a conceptually and mathematically useful property, as it is a conserved quantity. Several formulations of mechanics have been developed using energy as a core concept.
Work, a function of energy, is force times distance.
This says that the work (
The total energy of a system is sometimes called the Hamiltonian, after William Rowan Hamilton. The classical equations of motion can be written in terms of the Hamiltonian, even for highly complex or abstract systems. These classical equations have remarkably direct analogs in nonrelativistic quantum mechanics.[5]
Another energy-related concept is called the Lagrangian, after Joseph-Louis Lagrange. This formalism is as fundamental as the Hamiltonian, and both can be used to derive the equations of motion or be derived from them. It was invented in the context of classical mechanics, but is generally useful in modern physics. The Lagrangian is defined as the kinetic energy minus the potential energy. Usually, the Lagrange formalism is mathematically more convenient than the Hamiltonian for non-conservative systems (such as systems with friction).
Noether’s theorem (1918) states that any differentiable symmetry of the action of a physical system has a corresponding conservation law. Noether’s theorem has become a fundamental tool of modern theoretical physics and the calculus of variations. A generalisation of the seminal formulations on constants of motion in Lagrangian and Hamiltonian mechanics (1788 and 1833, respectively), it does not apply to systems that cannot be modeled with a Lagrangian; for example, dissipative systems with continuous symmetries need not have a corresponding conservation law.
Chemistry
In the context of chemistry, energy is an attribute of a substance as a consequence of its atomic, molecular, or aggregate structure. Since a chemical transformation is accompanied by a change in one or more of these kinds of structure, it is usually accompanied by a decrease, and sometimes an increase, of the total energy of the substances involved. Some energy may be transferred between the surroundings and the reactants in the form of heat or light; thus the products of a reaction have sometimes more but usually less energy than the reactants. A reaction is said to be exothermic or exergonic if the final state is lower on the energy scale than the initial state; in the less common case of endothermic reactions the situation is the reverse. Chemical reactions are usually not possible unless the reactants surmount an energy barrier known as the activation energy. The speed of a chemical reaction (at a given temperature T) is related to the activation energy E by the Boltzmann’s population factor e−E/kT; that is, the probability of a molecule to have energy greater than or equal to E at a given temperature T. This exponential dependence of a reaction rate on temperature is known as the Arrhenius equation. The activation energy necessary for a chemical reaction can be provided in the form of thermal energy.
Biology
In biology, energy is an attribute of all biological systems, from the biosphere to the smallest living organism. Within an organism it is responsible for growth and development of a biological cell or organelle of a biological organism. Energy used in respiration is stored in substances such as carbohydrates (including sugars), lipids, and proteins stored by cells. In human terms, the human equivalent (H-e) (Human energy conversion) indicates, for a given amount of energy expenditure, the relative quantity of energy needed for human metabolism, using as a standard an average human energy expenditure of 12,500 kJ per day and a basal metabolic rate of 80 watts. For example, if our bodies run (on average) at 80 watts, then a light bulb running at 100 watts is running at 1.25 human equivalents (100 ÷ 80) i.e. 1.25 H-e. For a difficult task of only a few seconds’ duration, a person can put out thousands of watts, many times the 746 watts in one official horsepower. For tasks lasting a few minutes, a fit human can generate perhaps 1,000 watts. For an activity that must be sustained for an hour, output drops to around 300; for an activity kept up all day, 150 watts is about the maximum.[6] The human equivalent assists understanding of energy flows in physical and biological systems by expressing energy units in human terms: it provides a «feel» for the use of a given amount of energy.[7]
Sunlight’s radiant energy is also captured by plants as chemical potential energy in photosynthesis, when carbon dioxide and water (two low-energy compounds) are converted into carbohydrates, lipids, proteins and oxygen. Release of the energy stored during photosynthesis as heat or light may be triggered suddenly by a spark in a forest fire, or it may be made available more slowly for animal or human metabolism when organic molecules are ingested and catabolism is triggered by enzyme action.
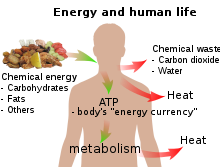
All living creatures rely on an external source of energy to be able to grow and reproduce – radiant energy from the Sun in the case of green plants and chemical energy (in some form) in the case of animals. The daily 1500–2000 Calories (6–8 MJ) recommended for a human adult are taken as food molecules, mostly carbohydrates and fats, of which glucose (C6H12O6) and stearin (C57H110O6) are convenient examples. The food molecules are oxidized to carbon dioxide and water in the mitochondria
and some of the energy is used to convert ADP into ATP:
ADP + HPO42− → ATP + H2O
The rest of the chemical energy of the carbohydrate or fat are converted into heat: the ATP is used as a sort of «energy currency», and some of the chemical energy it contains is used for other metabolism when ATP reacts with OH groups and eventually splits into ADP and phosphate (at each stage of a metabolic pathway, some chemical energy is converted into heat). Only a tiny fraction of the original chemical energy is used for work:[note 1]
- gain in kinetic energy of a sprinter during a 100 m race: 4 kJ
- gain in gravitational potential energy of a 150 kg weight lifted through 2 metres: 3 kJ
- Daily food intake of a normal adult: 6–8 MJ
It would appear that living organisms are remarkably inefficient (in the physical sense) in their use of the energy they receive (chemical or radiant energy); most machines manage higher efficiencies. In growing organisms the energy that is converted to heat serves a vital purpose, as it allows the organism tissue to be highly ordered with regard to the molecules it is built from. The second law of thermodynamics states that energy (and matter) tends to become more evenly spread out across the universe: to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe («the surroundings»).[note 2] Simpler organisms can achieve higher energy efficiencies than more complex ones, but the complex organisms can occupy ecological niches that are not available to their simpler brethren. The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ecology. As an example, to take just the first step in the food chain: of the estimated 124.7 Pg/a of carbon that is fixed by photosynthesis, 64.3 Pg/a (52%) are used for the metabolism of green plants,[8] i.e. reconverted into carbon dioxide and heat.
Earth sciences
In geology, continental drift, mountain ranges, volcanoes, and earthquakes are phenomena that can be explained in terms of energy transformations in the Earth’s interior,[9] while meteorological phenomena like wind, rain, hail, snow, lightning, tornadoes and hurricanes are all a result of energy transformations in our atmosphere brought about by solar energy.
Sunlight is the main input to Earth’s energy budget which accounts for its temperature and climate stability. Sunlight may be stored as gravitational potential energy after it strikes the Earth, as (for example when) water evaporates from oceans and is deposited upon mountains (where, after being released at a hydroelectric dam, it can be used to drive turbines or generators to produce electricity). Sunlight also drives most weather phenomena, save a few exceptions, like those generated by volcanic events for example. An example of a solar-mediated weather event is a hurricane, which occurs when large unstable areas of warm ocean, heated over months, suddenly give up some of their thermal energy to power a few days of violent air movement.
In a slower process, radioactive decay of atoms in the core of the Earth releases heat. This thermal energy drives plate tectonics and may lift mountains, via orogenesis. This slow lifting represents a kind of gravitational potential energy storage of the thermal energy, which may later be transformed into active kinetic energy during landslides, after a triggering event. Earthquakes also release stored elastic potential energy in rocks, a store that has been produced ultimately from the same radioactive heat sources. Thus, according to present understanding, familiar events such as landslides and earthquakes release energy that has been stored as potential energy in the Earth’s gravitational field or elastic strain (mechanical potential energy) in rocks. Prior to this, they represent release of energy that has been stored in heavy atoms since the collapse of long-destroyed supernova stars (which created these atoms).
Cosmology
In cosmology and astronomy the phenomena of stars, nova, supernova, quasars and gamma-ray bursts are the universe’s highest-output energy transformations of matter. All stellar phenomena (including solar activity) are driven by various kinds of energy transformations. Energy in such transformations is either from gravitational collapse of matter (usually molecular hydrogen) into various classes of astronomical objects (stars, black holes, etc.), or from nuclear fusion (of lighter elements, primarily hydrogen). The nuclear fusion of hydrogen in the Sun also releases another store of potential energy which was created at the time of the Big Bang. At that time, according to theory, space expanded and the universe cooled too rapidly for hydrogen to completely fuse into heavier elements. This meant that hydrogen represents a store of potential energy that can be released by fusion. Such a fusion process is triggered by heat and pressure generated from gravitational collapse of hydrogen clouds when they produce stars, and some of the fusion energy is then transformed into sunlight.
Quantum mechanics
In quantum mechanics, energy is defined in terms of the energy operator
(Hamiltonian) as a time derivative of the wave function. The Schrödinger equation equates the energy operator to the full energy of a particle or a system. Its results can be considered as a definition of measurement of energy in quantum mechanics. The Schrödinger equation describes the space- and time-dependence of a slowly changing (non-relativistic) wave function of quantum systems. The solution of this equation for a bound system is discrete (a set of permitted states, each characterized by an energy level) which results in the concept of quanta. In the solution of the Schrödinger equation for any oscillator (vibrator) and for electromagnetic waves in a vacuum, the resulting energy states are related to the frequency by Planck’s relation: 


Relativity
When calculating kinetic energy (work to accelerate a massive body from zero speed to some finite speed) relativistically – using Lorentz transformations instead of Newtonian mechanics – Einstein discovered an unexpected by-product of these calculations to be an energy term which does not vanish at zero speed. He called it rest energy: energy which every massive body must possess even when being at rest. The amount of energy is directly proportional to the mass of the body:
where
- m0 is the rest mass of the body,
- c is the speed of light in vacuum,
is the rest energy.
For example, consider electron–positron annihilation, in which the rest energy of these two individual particles (equivalent to their rest mass) is converted to the radiant energy of the photons produced in the process. In this system the matter and antimatter (electrons and positrons) are destroyed and changed to non-matter (the photons). However, the total mass and total energy do not change during this interaction. The photons each have no rest mass but nonetheless have radiant energy which exhibits the same inertia as did the two original particles. This is a reversible process – the inverse process is called pair creation – in which the rest mass of particles is created from the radiant energy of two (or more) annihilating photons.
In general relativity, the stress–energy tensor serves as the source term for the gravitational field, in rough analogy to the way mass serves as the source term in the non-relativistic Newtonian approximation.[10]
Energy and mass are manifestations of one and the same underlying physical property of a system. This property is responsible for the inertia and strength of gravitational interaction of the system («mass manifestations»), and is also responsible for the potential ability of the system to perform work or heating («energy manifestations»), subject to the limitations of other physical laws.
In classical physics, energy is a scalar quantity, the canonical conjugate to time. In special relativity energy is also a scalar (although not a Lorentz scalar but a time component of the energy–momentum 4-vector).[10] In other words, energy is invariant with respect to rotations of space, but not invariant with respect to rotations of spacetime (= boosts).
Transformation
| Type of transfer process | Description |
|---|---|
| Heat | equal amount of thermal energy in transit spontaneously towards a lower-temperature object |
| Work | equal amount of energy in transit due to a displacement in the direction of an applied force |
| Transfer of material | equal amount of energy carried by matter that is moving from one system to another |
A turbo generator transforms the energy of pressurized steam into electrical energy
Energy may be transformed between different forms at various efficiencies. Items that transform between these forms are called transducers. Examples of transducers include a battery (from chemical energy to electric energy), a dam (from gravitational potential energy to kinetic energy of moving water (and the blades of a turbine) and ultimately to electric energy through an electric generator), and a heat engine (from heat to work).
Examples of energy transformation include generating electric energy from heat energy via a steam turbine, or lifting an object against gravity using electrical energy driving a crane motor. Lifting against gravity performs mechanical work on the object and stores gravitational potential energy in the object. If the object falls to the ground, gravity does mechanical work on the object which transforms the potential energy in the gravitational field to the kinetic energy released as heat on impact with the ground. The Sun transforms nuclear potential energy to other forms of energy; its total mass does not decrease due to that itself (since it still contains the same total energy even in different forms) but its mass does decrease when the energy escapes out to its surroundings, largely as radiant energy.
There are strict limits to how efficiently heat can be converted into work in a cyclic process, e.g. in a heat engine, as described by Carnot’s theorem and the second law of thermodynamics. However, some energy transformations can be quite efficient. The direction of transformations in energy (what kind of energy is transformed to what other kind) is often determined by entropy (equal energy spread among all available degrees of freedom) considerations. In practice all energy transformations are permitted on a small scale, but certain larger transformations are not permitted because it is statistically unlikely that energy or matter will randomly move into more concentrated forms or smaller spaces.
Energy transformations in the universe over time are characterized by various kinds of potential energy, that has been available since the Big Bang, being «released» (transformed to more active types of energy such as kinetic or radiant energy) when a triggering mechanism is available. Familiar examples of such processes include nucleosynthesis, a process ultimately using the gravitational potential energy released from the gravitational collapse of supernovae to «store» energy in the creation of heavy isotopes (such as uranium and thorium), and nuclear decay, a process in which energy is released that was originally stored in these heavy elements, before they were incorporated into the Solar System and the Earth. This energy is triggered and released in nuclear fission bombs or in civil nuclear power generation. Similarly, in the case of a chemical explosion, chemical potential energy is transformed to kinetic and thermal energy in a very short time.
Yet another example is that of a pendulum. At its highest points the kinetic energy is zero and the gravitational potential energy is at its maximum. At its lowest point the kinetic energy is at its maximum and is equal to the decrease in potential energy. If one (unrealistically) assumes that there is no friction or other losses, the conversion of energy between these processes would be perfect, and the pendulum would continue swinging forever.
Energy is also transferred from potential energy (

|
|
(4) |
The equation can then be simplified further since 


Conservation of energy and mass in transformation
Energy gives rise to weight when it is trapped in a system with zero momentum, where it can be weighed. It is also equivalent to mass, and this mass is always associated with it. Mass is also equivalent to a certain amount of energy, and likewise always appears associated with it, as described in mass-energy equivalence. The formula E = mc², derived by Albert Einstein (1905) quantifies the relationship between relativistic mass and energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by J.J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass-energy equivalence#History for further information).
Part of the rest energy (equivalent to rest mass) of matter may be converted to other forms of energy (still exhibiting mass), but neither energy nor mass can be destroyed; rather, both remain constant during any process. However, since 

Reversible and non-reversible transformations
Thermodynamics divides energy transformation into two kinds: reversible processes and irreversible processes. An irreversible process is one in which energy is dissipated (spread) into empty energy states available in a volume, from which it cannot be recovered into more concentrated forms (fewer quantum states), without degradation of even more energy. A reversible process is one in which this sort of dissipation does not happen. For example, conversion of energy from one type of potential field to another is reversible, as in the pendulum system described above. In processes where heat is generated, quantum states of lower energy, present as possible excitations in fields between atoms, act as a reservoir for part of the energy, from which it cannot be recovered, in order to be converted with 100% efficiency into other forms of energy. In this case, the energy must partly stay as thermal energy and cannot be completely recovered as usable energy, except at the price of an increase in some other kind of heat-like increase in disorder in quantum states, in the universe (such as an expansion of matter, or a randomization in a crystal).
As the universe evolves with time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or as other kinds of increases in disorder). This has led to the hypothesis of the inevitable thermodynamic heat death of the universe. In this heat death the energy of the universe does not change, but the fraction of energy which is available to do work through a heat engine, or be transformed to other usable forms of energy (through the use of generators attached to heat engines), continues to decrease.
Conservation of energy
The fact that energy can be neither created nor destroyed is called the law of conservation of energy. In the form of the first law of thermodynamics, this states that a closed system’s energy is constant unless energy is transferred in or out as work or heat, and that no energy is lost in transfer. The total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system. Whenever one measures (or calculates) the total energy of a system of particles whose interactions do not depend explicitly on time, it is found that the total energy of the system always remains constant.[11]
While heat can always be fully converted into work in a reversible isothermal expansion of an ideal gas, for cyclic processes of practical interest in heat engines the second law of thermodynamics states that the system doing work always loses some energy as waste heat. This creates a limit to the amount of heat energy that can do work in a cyclic process, a limit called the available energy. Mechanical and other forms of energy can be transformed in the other direction into thermal energy without such limitations.[12] The total energy of a system can be calculated by adding up all forms of energy in the system.
Richard Feynman said during a 1961 lecture:[13]
There is a fact, or if you wish, a law, governing all natural phenomena that are known to date. There is no known exception to this law – it is exact so far as we know. The law is called the conservation of energy. It states that there is a certain quantity, which we call energy, that does not change in manifold changes which nature undergoes. That is a most abstract idea, because it is a mathematical principle; it says that there is a numerical quantity which does not change when something happens. It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number and when we finish watching nature go through her tricks and calculate the number again, it is the same.
Most kinds of energy (with gravitational energy being a notable exception)[14] are subject to strict local conservation laws as well. In this case, energy can only be exchanged between adjacent regions of space, and all observers agree as to the volumetric density of energy in any given space. There is also a global law of conservation of energy, stating that the total energy of the universe cannot change; this is a corollary of the local law, but not vice versa.[12][13]
This law is a fundamental principle of physics. As shown rigorously by Noether’s theorem, the conservation of energy is a mathematical consequence of translational symmetry of time,[15] a property of most phenomena below the cosmic scale that makes them independent of their locations on the time coordinate. Put differently, yesterday, today, and tomorrow are physically indistinguishable. This is because energy is the quantity which is canonical conjugate to time. This mathematical entanglement of energy and time also results in the uncertainty principle – it is impossible to define the exact amount of energy during any definite time interval (though this is practically significant only for very short time intervals). The uncertainty principle should not be confused with energy conservation – rather it provides mathematical limits to which energy can in principle be defined and measured.
Each of the basic forces of nature is associated with a different type of potential energy, and all types of potential energy (like all other types of energy) appear as system mass, whenever present. For example, a compressed spring will be slightly more massive than before it was compressed. Likewise, whenever energy is transferred between systems by any mechanism, an associated mass is transferred with it.
In quantum mechanics energy is expressed using the Hamiltonian operator. On any time scales, the uncertainty in the energy is by
which is similar in form to the Heisenberg Uncertainty Principle (but not really mathematically equivalent thereto, since H and t are not dynamically conjugate variables, neither in classical nor in quantum mechanics).
In particle physics, this inequality permits a qualitative understanding of virtual particles, which carry momentum. The exchange of virtual particles with real particles is responsible for the creation of all known fundamental forces (more accurately known as fundamental interactions). Virtual photons are also responsible for the electrostatic interaction between electric charges (which results in Coulomb’s law), for spontaneous radiative decay of excited atomic and nuclear states, for the Casimir force, for the Van der Waals force and some other observable phenomena.
Energy transfer
Closed systems
Energy transfer can be considered for the special case of systems which are closed to transfers of matter. The portion of the energy which is transferred by conservative forces over a distance is measured as the work the source system does on the receiving system. The portion of the energy which does not do work during the transfer is called heat.[note 3] Energy can be transferred between systems in a variety of ways. Examples include the transmission of electromagnetic energy via photons, physical collisions which transfer kinetic energy,[note 4] tidal interactions,[16] and the conductive transfer of thermal energy.
Energy is strictly conserved and is also locally conserved wherever it can be defined. In thermodynamics, for closed systems, the process of energy transfer is described by the first law:[note 5]
-
(1)
where 



-
(2)
This simplified equation is the one used to define the joule, for example.
Open systems
Beyond the constraints of closed systems, open systems can gain or lose energy in association with matter transfer (this process is illustrated by injection of an air-fuel mixture into a car engine, a system which gains in energy thereby, without addition of either work or heat). Denoting this energy by 
-
(3)
Thermodynamics
Internal energy
Internal energy is the sum of all microscopic forms of energy of a system. It is the energy needed to create the system. It is related to the potential energy, e.g., molecular structure, crystal structure, and other geometric aspects, as well as the motion of the particles, in form of kinetic energy. Thermodynamics is chiefly concerned with changes in internal energy and not its absolute value, which is impossible to determine with thermodynamics alone.[17]
First law of thermodynamics
The first law of thermodynamics asserts that the total energy of a system and its surroundings (but not necessarily thermodynamic free energy) is always conserved[18] and that heat flow is a form of energy transfer. For homogeneous systems, with a well-defined temperature and pressure, a commonly used corollary of the first law is that, for a system subject only to pressure forces and heat transfer (e.g., a cylinder-full of gas) without chemical changes, the differential change in the internal energy of the system (with a gain in energy signified by a positive quantity) is given as
,
where the first term on the right is the heat transferred into the system, expressed in terms of temperature T and entropy S (in which entropy increases and its change dS is positive when heat is added to the system), and the last term on the right hand side is identified as work done on the system, where pressure is P and volume V (the negative sign results since compression of the system requires work to be done on it and so the volume change, dV, is negative when work is done on the system).
This equation is highly specific, ignoring all chemical, electrical, nuclear, and gravitational forces, effects such as advection of any form of energy other than heat and PV-work. The general formulation of the first law (i.e., conservation of energy) is valid even in situations in which the system is not homogeneous. For these cases the change in internal energy of a closed system is expressed in a general form by
where 

Equipartition of energy
The energy of a mechanical harmonic oscillator (a mass on a spring) is alternately kinetic and potential energy. At two points in the oscillation cycle it is entirely kinetic, and at two points it is entirely potential. Over a whole cycle, or over many cycles, average energy is equally split between kinetic and potential. This is an example of the equipartition principle: the total energy of a system with many degrees of freedom is equally split among all available degrees of freedom, on average.
This principle is vitally important to understanding the behavior of a quantity closely related to energy, called entropy. Entropy is a measure of evenness of a distribution of energy between parts of a system. When an isolated system is given more degrees of freedom (i.e., given new available energy states that are the same as existing states), then total energy spreads over all available degrees equally without distinction between «new» and «old» degrees. This mathematical result is part of the second law of thermodynamics. The second law of thermodynamics is simple only for systems which are near or in a physical equilibrium state. For non-equilibrium systems, the laws governing the systems’ behavior are still debatable. One of the guiding principles for these systems is the principle of maximum entropy production.[19][20] It states that nonequilibrium systems behave in such a way as to maximize their entropy production.[21]
See also
- Combustion
- Energy democracy
- Index of energy articles
- Index of wave articles
- Orders of magnitude (energy)
- Power station
- Transfer energy
Notes
- ^ These examples are solely for illustration, as it is not the energy available for work which limits the performance of the athlete but the power output (in case of a sprinter) and the force (in case of a weightlifter).
- ^ Crystals are another example of highly ordered systems that exist in nature: in this case too, the order is associated with the transfer of a large amount of heat (known as the lattice energy) to the surroundings.
- ^ Although heat is «wasted» energy for a specific energy transfer (see: waste heat), it can often be harnessed to do useful work in subsequent interactions. However, the maximum energy that can be «recycled» from such recovery processes is limited by the second law of thermodynamics.
- ^ The mechanism for most macroscopic physical collisions is actually electromagnetic, but it is very common to simplify the interaction by ignoring the mechanism of collision and just calculate the beginning and end result.
- ^ There are several sign conventions for this equation. Here, the signs in this equation follow the IUPAC convention.
References
- ^ «Nuclear Energy | Definition, Formula & Examples | nuclear-power.com». Nuclear Power. Archived from the original on 2022-07-06. Retrieved 2022-07-06.
- ^ Harper, Douglas. «Energy». Online Etymology Dictionary. Archived from the original on October 11, 2007. Retrieved May 1, 2007.
- ^ Smith, Crosbie (1998). The Science of Energy – a Cultural History of Energy Physics in Victorian Britain. The University of Chicago Press. ISBN 978-0-226-76420-7.
- ^ Lofts, G; O’Keeffe D; et al. (2004). «11 – Mechanical Interactions». Jacaranda Physics 1 (2 ed.). Milton, Queensland, Australia: John Wiley & Sons Australia Ltd. p. 286. ISBN 978-0-7016-3777-4.
- ^ The Hamiltonian MIT OpenCourseWare website 18.013A Chapter 16.3 Accessed February 2007
- ^ «Retrieved on May-29-09». Uic.edu. Archived from the original on 2010-06-04. Retrieved 2010-12-12.
- ^ Bicycle calculator – speed, weight, wattage etc. «Bike Calculator». Archived from the original on 2009-05-13. Retrieved 2009-05-29..
- ^ Ito, Akihito; Oikawa, Takehisa (2004). «Global Mapping of Terrestrial Primary Productivity and Light-Use Efficiency with a Process-Based Model. Archived 2006-10-02 at the Wayback Machine» in Shiyomi, M. et al. (Eds.) Global Environmental Change in the Ocean and on Land. pp. 343–58.
- ^ «Earth’s Energy Budget». Okfirst.ocs.ou.edu. Archived from the original on 2008-08-27. Retrieved 2010-12-12.
- ^ a b Misner, Thorne, Wheeler (1973). Gravitation. San Francisco: W.H. Freeman. ISBN 978-0-7167-0344-0.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Berkeley Physics Course Volume 1. Charles Kittel, Walter D Knight and Malvin A Ruderman
- ^ a b The Laws of Thermodynamics Archived 2006-12-15 at the Wayback Machine including careful definitions of energy, free energy, et cetera.
- ^ a b Feynman, Richard (1964). The Feynman Lectures on Physics; Volume 1. US: Addison Wesley. ISBN 978-0-201-02115-8. Archived from the original on 2022-07-30. Retrieved 2022-05-04.
- ^ «E. Noether’s Discovery of the Deep Connection Between Symmetries and Conservation Laws». Physics.ucla.edu. 1918-07-16. Archived from the original on 2011-05-14. Retrieved 2010-12-12.
- ^ «Time Invariance». Ptolemy.eecs.berkeley.edu. Archived from the original on 2011-07-17. Retrieved 2010-12-12.
- ^ Jaffe, Robert L.; Taylor, Washington (2018). The Physics of Energy. Cambridge University Press. p. 611. ISBN 9781107016651. Archived from the original on 2022-07-30. Retrieved 2022-05-22.
- ^ I. Klotz, R. Rosenberg, Chemical Thermodynamics – Basic Concepts and Methods, 7th ed., Wiley (2008), p. 39
- ^ Kittel and Kroemer (1980). Thermal Physics. New York: W.H. Freeman. ISBN 978-0-7167-1088-2.
- ^ Onsager, L. (1931). «Reciprocal relations in irreversible processes». Phys. Rev. 37 (4): 405–26. Bibcode:1931PhRv…37..405O. doi:10.1103/PhysRev.37.405.
- ^ Martyushev, L.M.; Seleznev, V.D. (2006). «Maximum entropy production principle in physics, chemistry and biology». Physics Reports. 426 (1): 1–45. Bibcode:2006PhR…426….1M. doi:10.1016/j.physrep.2005.12.001.
- ^ Belkin, A.; et., al. (2015). «Self-Assembled Wiggling Nano-Structures and the Principle of Maximum Entropy Production». Sci. Rep. 5: 8323. Bibcode:2015NatSR…5E8323B. doi:10.1038/srep08323. PMC 4321171. PMID 25662746.
Further reading
- Alekseev, G.N. (1986). Energy and Entropy. Moscow: Mir Publishers.
- The Biosphere (A Scientific American Book), San Francisco, W.H. Freeman and Co., 1970, ISBN 0-7167-0945-7. This book, originally a 1970 Scientific American issue, covers virtually every major concern and concept since debated regarding materials and energy resources, population trends, and environmental degradation.
- Crowell, Benjamin (2011), «ch. 11», Light and Matter, Fullerton, California: Light and Matter, archived from the original on 2011-05-19, retrieved 2017-04-12
- Energy and Power (A Scientific American Book), San Francisco, W.H. Freeman and Co., 1971, ISBN 0-7167-0938-4.
- Ross, John S. (23 April 2002). «Work, Power, Kinetic Energy» (PDF). Project PHYSNET. Michigan State University. Archived (PDF) from the original on 26 April 2011. Retrieved 10 April 2009.
- Santos, Gildo M. «Energy in Brazil: a historical overview,» The Journal of Energy History (2018), online Archived 2019-02-09 at the Wayback Machine
- Smil, Vaclav (2008). Energy in nature and society: general energetics of complex systems. Cambridge, US: MIT Press. ISBN 978-0-262-19565-2.
- Walding, Richard; Rapkins, Greg; Rossiter, Glenn (1999). New Century Senior Physics. Melbourne, Australia: Oxford University Press. ISBN 978-0-19-551084-3.
Journals
- The Journal of Energy History / Revue d’histoire de l’énergie (JEHRHE), 2018–
External links
- Energy at Curlie
- Differences between Heat and Thermal energy Archived 2016-08-27 at the Wayback Machine – BioCab
Other forms: energies
The general meaning of energy is the ability to be active. If you have a lot of energy, it means you like to be active. If you plan a low-energy day, it means a day of lounging around.
Energy is from Greek energeia «activity, operation,» from energos «active, effective,» from en «in» plus ergon «work.» Specialized senses of the word energy include the power that comes from sources such as heat or electricity, and the ability of sources such as heat or light to do work. In physics, an erg is the centimeter-gram-second unit used to measure the amount of energy or the amount of work done.
Definitions of energy
-
“he plays tennis with great
energy”-
synonyms:
vigor, vigour, zip
-
noun
enterprising or ambitious drive
“Europeans often laugh at American
energy”-
synonyms:
get-up-and-go, push
-
noun
a healthy capacity for vigorous activity
“jogging works off my excess
energy”-
synonyms:
vim, vitality
see moresee less-
types:
-
juice
energetic vitality
-
ch’i, chi, ki, qi
the circulating life energy that in Chinese philosophy is thought to be inherent in all things; in traditional Chinese medicine the balance of negative and positive forms in the body is believed to be essential for good health
-
type of:
-
good health, healthiness
the state of being vigorous and free from bodily or mental disease
-
juice
-
noun
an imaginative lively style (especially style of writing)
“his writing conveys great
energy”-
synonyms:
muscularity, vigor, vigour, vim
-
noun
any source of usable power
“the DOE is responsible for maintaining the
energy policy” -
noun
(physics) a thermodynamic quantity equivalent to the capacity of a physical system to do work; the units of energy are joules or ergs
“energy can take a wide variety of forms”
-
synonyms:
free energy
see moresee less-
types:
- show 32 types…
- hide 32 types…
-
activation energy, energy of activation
the energy that an atomic system must acquire before a process (such as an emission or reaction) can occur
-
alternative energy
energy derived from sources that do not use up natural resources or harm the environment
-
atomic energy, nuclear energy
the energy released by a nuclear reaction
-
binding energy, separation energy
the energy required to separate particles from a molecule or atom or nucleus; equals the mass defect
-
chemical energy
that part of the energy in a substance that can be released by a chemical reaction
-
electrical energy, electricity
energy made available by the flow of electric charge through a conductor
-
energy level, energy state
a definite stable energy that a physical system can have; used especially of the state of electrons in atoms or molecules
-
rest energy
the energy equivalent to the mass of a particle at rest in an inertial frame of reference; equal to the rest mass times the square of the speed of light
-
work
(physics) a manifestation of energy; the transfer of energy from one physical system to another expressed as the product of a force and the distance through which it moves a body in the direction of that force
-
heat, heat energy
a form of energy that is transferred by a difference in temperature
-
mechanical energy
energy in a mechanical form
-
radiant energy
energy that is transmitted in the form of (electromagnetic) radiation; energy that exists in the absence of matter
-
radiation
energy that is radiated or transmitted in the form of rays or waves or particles
-
AC, alternating current, alternating electric current
an electric current that reverses direction sinusoidally
-
atomic power, nuclear power
nuclear energy regarded as a source of electricity for the power grid (for civilian use)
-
ionizing radiation
high-energy radiation capable of producing ionization in substances through which it passes
-
cosmic radiation
radiation coming from outside the solar system
-
DC, direct current, direct electric current
an electric current that flows in one direction steadily
-
signal
an electric quantity (voltage or current or field strength) whose modulation represents coded information about the source from which it comes
-
electromagnetic radiation, electromagnetic wave, nonparticulate radiation
radiation consisting of waves of energy associated with electric and magnetic fields resulting from the acceleration of an electric charge
-
geothermal energy
energy derived from the heat in the interior of the earth
-
K.E., kinetic energy
the mechanical energy that a body has by virtue of its motion
-
heat of dissociation
the heat required for a fluid substance to break up into simpler constituents
-
heat of formation
the heat evolved or absorbed during the formation of one mole of a substance from its component elements
-
heat of solution
the heat evolved or absorbed when one mole of a substance is dissolved in a large volume of a solvent
-
heat of transformation, latent heat
heat absorbed or radiated during a change of phase at a constant temperature and pressure
-
specific heat
the heat required to raise the temperature of one gram of a substance one degree centigrade
-
luminous energy
the energy associated with visible light
-
P.E., potential energy
the mechanical energy that a body has by virtue of its position; stored energy
-
solar energy, solar power
energy from the sun that is converted into thermal or electrical energy
-
solar radiation
radiation from the sun
-
wind generation, wind power
power derived from the wind (as by windmills)
-
type of:
-
physical phenomenon
a natural phenomenon involving the physical properties of matter and energy
DISCLAIMER: These example sentences appear in various news sources and books to reflect the usage of the word ‘energy’.
Views expressed in the examples do not represent the opinion of Vocabulary.com or its editors.
Send us feedback
EDITOR’S CHOICE
Look up energy for the last time
Close your vocabulary gaps with personalized learning that focuses on teaching the
words you need to know.
Sign up now (it’s free!)
Whether you’re a teacher or a learner, Vocabulary.com can put you or your class on the path to systematic vocabulary improvement.
Get started
Dr. Karl offers compelling counter-arguments to some of the speculations of the BPP, although he points us toward the controversial energy source of * zero point energy* as the breakthrough discovery we can use to power our starships. ❋ Unknown (2004)
Rather, conventional energy strategies adopt the energy trickle-down approach to social welfare and implicitly assume that if energy supplies are increased, these problems will take care of themselves. ❋ Unknown (2000)
Take, for example, the two most important terms — the energy released by the fire and the energy recovered by the pot contents (energy utilised in the literature). ❋ Unknown (1989)
Since the energy possessed by coal only becomes available when the coal is made to undergo a chemical change, it is sometimes called _chemical energy_. ❋ William McPherson (N/A)
Now when this material is reduced by the process of digestion to simpler bodies with fewer molecules, such as carbon dioxid, urea, and water, the force stored up in the meat as potential energy becomes manifest and is used as active life-force known as _kinetic energy_. ❋ Albert F. Blaisdell (N/A)
An important fact about energy is, that all energy _tends to take the form of heat energy_. ❋ J. Arthur Thomson (1897)
It must, one would think, have been the badness of the ` ` copy » that induced the compositors to turn ` ` the nature and theory of the Greek verb » into _the native theology of the Greek verb_; ` ` the conser < p 124 > vation of energy » into the _conversation of energy_; and the ` ` Forest Conservancy ❋ Henry Benjamin Wheatley (1877)
DeSmog uncovered information that two of the three directors on the board of the Natural Resources Stewardship Project are registered energy industry lobbyists and senior executives of the High Park Advocacy Group, a Toronto-based lobby firm that specializes in �energy, environment and ethics. ❋ Unknown (2009)
It is possible that the two states are similar to the difference between potential and kinetic energy; and we must remember that _energy is always noticed or experienced by us, as energy, in its expenditure, never in its accumulation_. [ ❋ Hereward Carrington (1919)
In an ideal situation where you put food in, and all energy is spent cooling it and not random amounts of air, there wouldn’t be a sizable difference in energy usage between a stocked and near empty freezer. ❋ Unknown (2009)
In Western culture and medicine, the word energy connotes something New Age but not tangible or real. ❋ Tami Lynn Kent (2011)
A technological breakthrough in energy is very different. ❋ Unknown (2010)
Also be aware that I use the term energy a lot, but you shouldn’t take it too literally. ❋ J. Steven York (2009)
The ability of the channel to strip the potassium ion of its water and allow it to pass at no cost in energy is a kind of selective catalysed ion transport. ❋ Unknown (2003)
The Greek word for effective is the word from which we get our term energy. ❋ BOB RUSSELL (2003)
The term energy services is used to describe these benefits, which in households include illumination, cooked food, comfortable indoor temperatures, refrigeration, and transportation. ❋ Unknown (2000)
Renewed concern about abortion rights may drain energy from the anti-porn movement. ❋ Unknown (1992)
1) «Dang, that girl just brought a 2-litre bottle of soda to school, and now she’s just [hangin] in the back of class with her [earbuds] in. I wish I could channel that energy.»
2) «Bro, I just hung out with the [theatre kids], and I swear, they radiate this energy that nobody else can.» ❋ CoolMeme (2020)
When I was [babysitting], the kid got a sudden [surge] of energy, and I will never feed that kid [ice cream] again. ❋ Imaprettycoolbrunette (2005)
[Dwayne] was throwin’ hella ENERGY [my way].I know he’s down for [the get down]! ❋ CMACK7 (2007)
I was [talking with] [Ryan] and his energy [isn’t] the same anymore. ❋ Axvy.o (2020)
If its energy between us [im gonna] cut.
[These dudes] talking [noise] its gonna be energy. ❋ Houmane (2007)
I so [munch] [ENergy] i can go [all night] ❋ Energy (1999)
Inquisitive Child: Oh, wiser, older, more intelligent [connoisseur] of music, what’s a genuinely good, non-whiny band that has really cool guys in it that are just too adorable for words?
[Connoisseur] of Music: Oh dear, sweet, [naiive] [young one], simply go to www.myspace.com/thisisenergy, and your question will be answered. (This Is Energy) ❋ Connoisseur_of_music221 (2009)
-Did you [hear] [This is Energy]?
-Yeah, it [rocks]! ❋ Justina_K (2009)
Steve : Yeah, I’m a broke loser.
[Jose] : Call [Jeremy], he’s got that energi thing going on.
Steve : Sweet, he’ll get us [set up]. ❋ Mya Smith (2004)
Typing like this, with perfect grammar and [punctuation] produces a specific energy.
typing like this with [bad grammar] and no punctuation has a different energyor even with no [capitalization], big words, and proper punctuation and even a period at the end produces a uniquely different energy. ❋ Hudzell (2020)


- Entertainment & Pop Culture
- Geography & Travel
- Health & Medicine
- Lifestyles & Social Issues
- Literature
- Philosophy & Religion
- Politics, Law & Government
- Science
- Sports & Recreation
- Technology
- Visual Arts
- World History
- On This Day in History
- Quizzes
- Podcasts
- Dictionary
- Biographies
- Summaries
- Top Questions
- Infographics
- Demystified
- Lists
- #WTFact
- Companions
- Image Galleries
- Spotlight
- The Forum
- One Good Fact
- Entertainment & Pop Culture
- Geography & Travel
- Health & Medicine
- Lifestyles & Social Issues
- Literature
- Philosophy & Religion
- Politics, Law & Government
- Science
- Sports & Recreation
- Technology
- Visual Arts
- World History
- Britannica Explains
In these videos, Britannica explains a variety of topics and answers frequently asked questions. - Britannica Classics
Check out these retro videos from Encyclopedia Britannica’s archives. - Demystified Videos
In Demystified, Britannica has all the answers to your burning questions. - #WTFact Videos
In #WTFact Britannica shares some of the most bizarre facts we can find. - This Time in History
In these videos, find out what happened this month (or any month!) in history.
- Student Portal
Britannica is the ultimate student resource for key school subjects like history, government, literature, and more. - COVID-19 Portal
While this global health crisis continues to evolve, it can be useful to look to past pandemics to better understand how to respond today. - 100 Women
Britannica celebrates the centennial of the Nineteenth Amendment, highlighting suffragists and history-making politicians. - Saving Earth
Britannica Presents Earth’s To-Do List for the 21st Century. Learn about the major environmental problems facing our planet and what can be done about them! - SpaceNext50
Britannica presents SpaceNext50, From the race to the Moon to space stewardship, we explore a wide range of subjects that feed our curiosity about space!
1
b
: the capacity of acting or being active
c
: a usually positive spiritual force
the energy flowing through all people
2
: vigorous exertion of power : effort
investing time and energy
3
: a fundamental entity of nature that is transferred between parts of a system in the production of physical change within the system and usually regarded as the capacity for doing work
4
: usable power (such as heat or electricity)
also
: the resources for producing such power
Synonyms
Choose the Right Synonym for energy
the awesome power of flowing water
force implies the actual effective exercise of power.
used enough force to push the door open
energy applies to power expended or capable of being transformed into work.
a worker with boundless energy
strength applies to the quality or property of a person or thing that makes possible the exertion of force or the withstanding of strain, pressure, or attack.
use weight training to build your strength
might implies great or overwhelming power or strength.
the belief that might makes right
Example Sentences
The kids are always so full of energy.
They devoted all their energy to the completion of the project.
They devoted all their energies to the completion of the project.
She puts a lot of energy into her work.
The newer appliances conserve more energy.
Recent Examples on the Web
While coffee is best known for waking us up, elevating alertness and giving us an extra burst of energy.
—
Officials say the purpose of the alliance is to expand the access and use of renewable energy at a competitive price.
—
But for Sperberg, sapped of energy and under siege from acid reflux, the walk was arduous.
—
Baltimore also will get a lot of energy from 21-year-old Gunnar Henderson, who will eventually take over an infield spot.
—
Your time and energy are valuable, nonregenerative assets as well.
—
Put the time and energy into doing something productive that will materially benefit the world (or, less idealistically, your business) instead.
—
Some cities use as much as 60% of their energy on these utilities!
—
Enlarge Microsoft Microsoft has spent a lot of time and energy over the last few months adding generative AI features to all its products, particularly its long-standing, long-struggling Bing search engine.
—
See More
These examples are programmatically compiled from various online sources to illustrate current usage of the word ‘energy.’ Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Etymology
Late Latin energia, from Greek energeia activity, from energos active, from en in + ergon work — more at work
First Known Use
1783, in the meaning defined at sense 1a
Time Traveler
The first known use of energy was
in 1783
Dictionary Entries Near energy
Cite this Entry
“Energy.” Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/energy. Accessed 14 Apr. 2023.
Share
More from Merriam-Webster on energy
Last Updated:
7 Apr 2023
— Updated example sentences
Subscribe to America’s largest dictionary and get thousands more definitions and advanced search—ad free!
Merriam-Webster unabridged

The concept of energy is key to science and engineering. Here is the definition, examples of energy, and a look at the way it is classified.
Energy Definition
In science, energy is the ability to do work or heat objects. It is a scalar physical quantity, which means it has magnitude, but no direction. Energy is conserved, which means it can change from one form to another, but isn’t created or destroyed. There are many different types of energy, such as kinetic energy, potential energy, light, sound, and nuclear energy.
Word Origin and Units
The term “energy” comes from the Greek word energeia or from the French words enmeaning in and ergon which means work. The SI unit of energy is the joule (J), where 1 J = 1kg⋅m2⋅s−2. Other units include the kilowatt-hour (kW-h), British thermal unit (BTU), calorie (c), kilocalorie (C), electron-volt (EV), erg, and foot-pound (ft-lb).
What Losing Energy Means
One form of energy may be converted into another without violating a law of thermodynamics. Not all of these forms of energy are equally useful for practical applications. When energy is “lost”, it means the energy can’t be recaptured for use. This usually occurs when heat is produced. Losing energy doesn’t mean there is less of it, only that it has changed forms.
Energy may be either renewable or nonrenewable. Photosynthesis is an example of a process the produces renewable energy. Burning coal is an example of nonrenewable energy. The plant continues to produce chemical energy in the form of sugar, by converting solar energy. Once coal is burned, the ash can’t be used to continue the reaction.
Kinetic Energy and Potential Energy
The various forms of energy are classified as kinetic energy, potential energy, or a mixture of them. Kinetic energy is energy of motion, while potential energy is stored energy or energy of position. The total of the sum of the kinetic and potential energy of a system is constant, but energy changes from one form to another.
For example, when you hold an apple motionless above the ground, it has potential energy, but no kinetic energy. When you drop the apple, it has both kinetic and potential energy as it falls. Just before it strikes the ground, it has maximum kinetic energy, but no potential energy.
Renewable and Non-Renewable Energy
Another broad way of classifying energy is as renewable or non-renewable. Renewable energy is energy that replenishes within a human lifetime. Examples include solar energy, wind energy, and biomass. Non-renewable energy either does not regenerate or else takes longer than a human lifespan to do so. Fossil fuels are an example of non-renewable energy.
Forms of Energy
There are many different forms energy can take. Here are some examples:
- nuclear energy – energy released by changes in the atomic nucleus, such as fission or fusion
- electrical energy – energy based on the attraction, repulsion, and movement of electrical charge, such as electrons, protons, or ions
- chemical energy – energy based on the difference between the amount required to form chemical bonds versus how much is needed to break them
- mechanical energy – the sum of the translational and rotational kinetic and potential energies of a system
- gravitational energy – energy stored in gravitational fields
- ionization energy – energy that binds an electron to its atom or molecule
- magnetic energy – energy stored within magnetic fields
- elastic energy – energy of a material that causes it to return to its original shape if it’s deformed
- radiant energy – electromagnetic radiation, such as light from the sun or heat from a stove
- thermal energy – kinetic energy due to the motion of subatomic particles, atoms, and molecules
Examples of Energy
Here are some everyday examples of energy and a look at the types of energy:
- Throwing a ball: Throwing a ball is an example of kinetic energy, potential energy, and mechanical energy
- Fire: Fire is thermal energy, chemical energy, and radiant energy. Its source may be either renewable (wood) or non-renewable (coal).
- Charging a phone battery: Charging a phone involves electrical energy, chemical energy (for the battery), and both kinetic and potential energy. The stored electrical charge is potential energy, while moving charge is kinetic energy.
References
- Harper, Douglas. “Energy”. Online Etymology Dictionary.
- Smith, Crosbie (1998). The Science of Energy – a Cultural History of Energy Physics in Victorian Britain. The University of Chicago Press. ISBN 978-0-226-76420-7.
энергия, сила, силы, мощность, энергичность
существительное ↓
- энергия; сила
a man of indomitable energy — человек неукротимой энергии
to work with energy — работать энергично
through their own energy and enterprise — благодаря собственной энергии и предприимчивости
- pl. усилия, активность, деятельность
to apply /to devote/ one’s energies — приложить усилия
to brace one’s energies — собраться с духом
- физ., тех. энергия
electrical energy — электроэнергия
potential [kinetic /motive/, static, latent] energy — потенциальная [кинетическая, статистическая, скрытая] энергия
- тех. энергетика
Мои примеры
Словосочетания
renewable energy such as solar power — энергия из возобновляемых источников, например, солнечная 
a man of energy and commitment — человек энергичный и преданный делу 
a failure traceable to lack of energy — неудача, которую можно отнести на счёт недостатка сил 
signs of the decrement of vital energy — признаки понижения жизненной активности 
electric energy — электроэнергия 
electrical field energy — энергетика электрического поля 
to emit energy — выделять энергию 
to provide energy for smb. — обеспечивать энергией кого-л. 
to harness energy — использовать энергию 
atomic, nuclear energy — атомная энергия 
sources of energy — источники энергии 
energy crisis — энергетический кризис 
Примеры с переводом
All her energy was focused upon her children. 
Вся её энергия была сосредоточена на детях.
The solar panels store energy. 
Солнечные батареи накапливают энергию.
How much energy can this battery store? 
Какова энергоёмкость этой батареи?
Federal Energy Administration 
Федеральное энергетическое управление (в США)
International Atomic Energy Agency 
Международное агентство по атомной энергии (МАГАТЭ)
Helping people takes time and energy. 
Помощь людям отнимает много времени и сил.
Atomic Energy Authority 
Управление атомной энергетики (в Великобритании)
ещё 23 примера свернуть
Примеры, ожидающие перевода
The boy has a lot of nervous energy. 
all technology and energy revving up for the greatest clash of arms in history. 
… all technology and energy revving up for the greatest clash of arms in history. 
Для того чтобы добавить вариант перевода, кликните по иконке ☰, напротив примера.
Возможные однокоренные слова
energize — возбуждать, питать энергией, пропускать ток, сообщать энергию
energies — силы
high-energy — высококалорийный, высокоэнергетический, с высокой энергией, высокой энергии
low-energy — низкоэнергетический, с малым потреблением энергии, экономичный
bioenergy — энергия биологических процессов, энергия, получаемая из биотоплива, биоэнергия
macroenergy — макроскопическое количество энергии, макроэнергия
Формы слова
noun
ед. ч.(singular): energy
мн. ч.(plural): energies
- Top Definitions
- Synonyms
- Quiz
- Related Content
- More About Energy
- Examples
- British
- Scientific
- Cultural
This shows grade level based on the word’s complexity.
[ en-er-jee ]
/ ˈɛn ər dʒi /
This shows grade level based on the word’s complexity.
noun, plural en·er·gies.
the capacity for vigorous activity; available power: I eat chocolate to get quick energy.
an adequate or abundant amount of such power: I seem to have no energy these days.
Often energies. a feeling of tension caused or seeming to be caused by an excess of such power: to work off one’s energies at tennis.
an exertion of such power: She plays tennis with great energy.
the habit of vigorous activity; vigor as a characteristic: Foreigners both admire and laugh at American energy.
the ability to act, lead others, effect, etc., forcefully.
forcefulness of expression: a writing style abounding with energy.
Physics. the capacity to do work; the property of a system that diminishes when the system does work on any other system, by an amount equal to the work so done; potential energy. Symbol: E
any source of usable power, as fossil fuel, electricity, or solar radiation.
QUIZ
CAN YOU ANSWER THESE COMMON GRAMMAR DEBATES?
There are grammar debates that never die; and the ones highlighted in the questions in this quiz are sure to rile everyone up once again. Do you know how to answer the questions that cause some of the greatest grammar debates?
Which sentence is correct?
Origin of energy
1575–85; <Late Latin energīa<Greek enérgeia activity, equivalent to energe- (stem of energeîn to be active; see en-2, work) + -ia-y3
OTHER WORDS FROM energy
hy·per·en·er·gy, nounself-en·er·gy, noun
Words nearby energy
energid, energism, energize, energizer, energumen, energy, energy audit, energy band, energy bar, energy conversion, energy crop
Dictionary.com Unabridged
Based on the Random House Unabridged Dictionary, © Random House, Inc. 2023
MORE ABOUT ENERGY
What is energy?
Energy refers to available power or motivation to move, as in Jada found that getting enough sleep each night gave her the energy to live each day.
Energy also refers to power that is used with exertion or force, as in Monique brought energy to the team, leading them to win more games.
In physics, energy is the power or heat that is created when something moves, is burned, or is exerted. It is typically represented in two forms: potential and kinetic energy. Potential energy is power that is stored in something as it sits still or is unburned. For example, coal contains a large amount of potential energy that is released when the coal is burned. As the coal burns, that potential energy becomes kinetic energy, energy related to the particles in the system.
Energy is a common word with several other senses related to power or motivation.
Example: Darryl found out the hard way that cell phone batteries lose their energy in the cold.
Where does energy come from?
The first records of the term energy come from the late 1500s. It ultimately comes from the Greek term energeîn, meaning “to be active.” Activity can come in many forms, but almost all burn energy.
Potential and kinetic energy can be applied to humans, too. As you eat and sleep, you build up potential energy, and as you physically move, think, breathe, or perform any physical action, that energy is used kinetically. When you’re tired, you might say you’re low on energy. And when you decide to put your energies into your art, you are spending more time doing your art and, as a result, spending more of your energy on it.
Did you know … ?
How is energy used in real life?
Energy is a common word used both in the scientific sense and in other senses, particularly those related to the power we or our devices have or don’t have.
U attract the energy u put out ..
— TRAVIS SCOTT (@trvisXX) October 2, 2018
I’m tired of all the negative energy in the world
— marshmello (@marshmello) May 31, 2018
We need to work harder & longer hours to catch up with the world. Fewer holidays, better teachers but above all sort the energy shortage.
— Reham Khan (@RehamKhan1) April 22, 2016
Try using energy!
Is energy used correctly in the following sentence?
When Quinn focused his energies on his school work, his grades went up.
Words related to energy
efficiency, intensity, power, spirit, stamina, strength, toughness, vitality, dynamism, electricity, heat, potential, service, activity, animation, application, ardor, birr, dash, drive
How to use energy in a sentence
-
Some of that energy enters the water, and when it does, the seismic waves slow down, becoming T waves.
-
Launched in 2015, the project’s purpose is to determine the feasibility of underwater data centers powered by offshore renewable energy.
-
This energy, “orgone,” was supposedly a life-force of sorts.
-
This represents a revolutionary shift in our ability to capture solar energy in real time rather than being dependent on solar energy of the past.
-
Yet negotiations over the final shape of a deal are set to be fraught amid national differences in wealth, energy sources and industrial strength.
-
I think a lot of it has to do with the attitude and the energy behind it and the honesty.
-
Total oil production figures include crude oil, natural gas liquids, and other liquid energy products.
-
The energy economy has always been a fixture of Texas life, and that has not changed.
-
Day by day, it drives people to distraction by diverting energy to mindless legal compliance.
-
Chickens require significantly less land, water, and energy than all other meat options except farmed salmon.
-
This is the first and principal point at which we can stanch the wastage of teaching energy that now goes on.
-
Sleek finds it far harder work than fortune-making; but he pursues his Will-o’-the-Wisp with untiring energy.
-
This may be done by taking the humming tone and bringing to bear upon it a strong pressure of energy.
-
It was, of course, the suppressed emotional energy finding another outlet.
-
She was putting her papers tidy again with calm fingers, while his own were almost cramped with the energy of suppressed desire.
British Dictionary definitions for energy
noun plural -gies
intensity or vitality of action or expression; forcefulness
capacity or tendency for intense activity; vigour
vigorous or intense action; exertion
physics
- the capacity of a body or system to do work
- a measure of this capacity, expressed as the work that it does in changing to some specified reference state. It is measured in joules (SI units)Symbol: E
Word Origin for energy
C16: from Late Latin energīa, from Greek energeia activity, from energos effective, from en- ² + ergon work
Collins English Dictionary — Complete & Unabridged 2012 Digital Edition
© William Collins Sons & Co. Ltd. 1979, 1986 © HarperCollins
Publishers 1998, 2000, 2003, 2005, 2006, 2007, 2009, 2012
Scientific definitions for energy
The capacity or power to do work, such as the capacity to move an object (of a given mass) by the application of force. Energy can exist in a variety of forms, such as electrical, mechanical, chemical, thermal, or nuclear, and can be transformed from one form to another. It is measured by the amount of work done, usually in joules or watts. See also conservation of energy kinetic energy potential energy. Compare power work.
The American Heritage® Science Dictionary
Copyright © 2011. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.
Cultural definitions for energy
notes for energy
The most important property of energy is that it is conserved — that is, the total energy of an isolated system does not change with time. This is known as the law of conservation of energy. Energy can, however, change form; for example, it can be turned into mass and back again into energy.
The New Dictionary of Cultural Literacy, Third Edition
Copyright © 2005 by Houghton Mifflin Harcourt Publishing Company. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.