This article is about the state of matter. For liquified petroleum gas used as an automotive fuel, see autogas. For gasoline («gas»), see gasoline. For the uses of gases, and other meanings, see Gas (disambiguation).
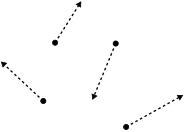
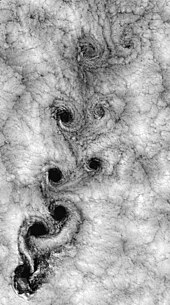
Drifting smoke particles indicate the movement of the surrounding gas.
Gas is one of the four fundamental states of matter. The others are solid, liquid, and plasma.[1]
A pure gas may be made up of individual atoms (e.g. a noble gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). A gas mixture, such as air, contains a variety of pure gases. What distinguishes a gas from liquids and solids is the vast separation of the individual gas particles. This separation usually makes a colourless gas invisible to the human observer.
The gaseous state of matter occurs between the liquid and plasma states,[2] the latter of which provides the upper temperature boundary for gases. Bounding the lower end of the temperature scale lie degenerative quantum gases[3] which are gaining increasing attention.[4]
High-density atomic gases super-cooled to very low temperatures are classified by their statistical behavior as either Bose gases or Fermi gases. For a comprehensive listing of these exotic states of matter see list of states of matter.
Elemental gases[edit]
The only chemical elements that are stable diatomic homonuclear molecular gases at STP are hydrogen (H2), nitrogen (N2), oxygen (O2), and two halogens: fluorine (F2) and chlorine (Cl2). When grouped together with the monatomic noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – these gases are referred to as «elemental gases».
Etymology[edit]
The word gas was first used by the early 17th-century Flemish chemist Jan Baptist van Helmont.[5] He identified carbon dioxide, the first known gas other than air.[6] Van Helmont’s word appears to have been simply a phonetic transcription of the Ancient Greek word χάος ‘chaos‘ – the g in Dutch being pronounced like ch in «loch» (voiceless velar fricative, ) – in which case Van Helmont was simply following the established alchemical usage first attested in the works of Paracelsus. According to Paracelsus’s terminology, chaos meant something like ‘ultra-rarefied water‘.[7]
An alternative story is that Van Helmont’s term was derived from «gahst (or geist), which signifies a ghost or spirit».[8] That story is given no credence by the editors of the Oxford English Dictionary.[9] In contrast, the French-American historian Jacques Barzun speculated that Van Helmont had borrowed the word from the German Gäscht, meaning the froth resulting from fermentation.[10]
Physical characteristics[edit]
Because most gases are difficult to observe directly, they are described through the use of four physical properties or macroscopic characteristics: pressure, volume, number of particles (chemists group them by moles) and temperature. These four characteristics were repeatedly observed by scientists such as Robert Boyle, Jacques Charles, John Dalton, Joseph Gay-Lussac and Amedeo Avogadro for a variety of gases in various settings. Their detailed studies ultimately led to a mathematical relationship among these properties expressed by the ideal gas law (see simplified models section below).
Gas particles are widely separated from one another, and consequently, have weaker intermolecular bonds than liquids or solids. These intermolecular forces result from electrostatic interactions between gas particles. Like-charged areas of different gas particles repel, while oppositely charged regions of different gas particles attract one another; gases that contain permanently charged ions are known as plasmas. Gaseous compounds with polar covalent bonds contain permanent charge imbalances and so experience relatively strong intermolecular forces, although the molecule while the compound’s net charge remains neutral. Transient, randomly induced charges exist across non-polar covalent bonds of molecules and electrostatic interactions caused by them are referred to as Van der Waals forces. The interaction of these intermolecular forces varies within a substance which determines many of the physical properties unique to each gas.[11][12] A comparison of boiling points for compounds formed by ionic and covalent bonds leads us to this conclusion.[13] The drifting smoke particles in the image provides some insight into low-pressure gas behavior.
Compared to the other states of matter, gases have low density and viscosity. Pressure and temperature influence the particles within a certain volume. This variation in particle separation and speed is referred to as compressibility. This particle separation and size influences optical properties of gases as can be found in the following list of refractive indices. Finally, gas particles spread apart or diffuse in order to homogeneously distribute themselves throughout any container.
Macroscopic view of gases[edit]
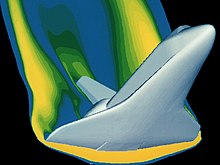
Shuttle imagery of re-entry phase
When observing a gas, it is typical to specify a frame of reference or length scale. A larger length scale corresponds to a macroscopic or global point of view of the gas. This region (referred to as a volume) must be sufficient in size to contain a large sampling of gas particles. The resulting statistical analysis of this sample size produces the «average» behavior (i.e. velocity, temperature or pressure) of all the gas particles within the region. In contrast, a smaller length scale corresponds to a microscopic or particle point of view.
Macroscopically, the gas characteristics measured are either in terms of the gas particles themselves (velocity, pressure, or temperature) or their surroundings (volume). For example, Robert Boyle studied pneumatic chemistry for a small portion of his career. One of his experiments related the macroscopic properties of pressure and volume of a gas. His experiment used a J-tube manometer which looks like a test tube in the shape of the letter J. Boyle trapped an inert gas in the closed end of the test tube with a column of mercury, thereby making the number of particles and the temperature constant. He observed that when the pressure was increased in the gas, by adding more mercury to the column, the trapped gas’ volume decreased (this is known as an inverse relationship). Furthermore, when Boyle multiplied the pressure and volume of each observation, the product was constant. This relationship held for every gas that Boyle observed leading to the law, (PV=k), named to honor his work in this field.
There are many mathematical tools available for analyzing gas properties. As gases are subjected to extreme conditions, these tools become more complex, from the Euler equations for inviscid flow to the Navier–Stokes equations[14] that fully account for viscous effects. These equations are adapted to the conditions of the gas system in question. Boyle’s lab equipment allowed the use of algebra to obtain his analytical results. His results were possible because he was studying gases in relatively low pressure situations where they behaved in an «ideal» manner. These ideal relationships apply to safety calculations for a variety of flight conditions on the materials in use. The high technology equipment in use today was designed to help us safely explore the more exotic operating environments where the gases no longer behave in an «ideal» manner. This advanced math, including statistics and multivariable calculus, makes possible the solution to such complex dynamic situations as space vehicle reentry. An example is the analysis of the space shuttle reentry pictured to ensure the material properties under this loading condition are appropriate. In this flight regime, the gas is no longer behaving ideally.
Pressure[edit]
The symbol used to represent pressure in equations is «p» or «P» with SI units of pascals.
When describing a container of gas, the term pressure (or absolute pressure) refers to the average force per unit area that the gas exerts on the surface of the container. Within this volume, it is sometimes easier to visualize the gas particles moving in straight lines until they collide with the container (see diagram at top of the article). The force imparted by a gas particle into the container during this collision is the change in momentum of the particle.[15] During a collision only the normal component of velocity changes. A particle traveling parallel to the wall does not change its momentum. Therefore, the average force on a surface must be the average change in linear momentum from all of these gas particle collisions.
Pressure is the sum of all the normal components of force exerted by the particles impacting the walls of the container divided by the surface area of the wall.
Temperature[edit]
Air balloon shrinks after submersion in liquid nitrogen
The symbol used to represent temperature in equations is T with SI units of kelvins.
The speed of a gas particle is proportional to its absolute temperature. The volume of the balloon in the video shrinks when the trapped gas particles slow down with the addition of extremely cold nitrogen. The temperature of any physical system is related to the motions of the particles (molecules and atoms) which make up the [gas] system.[16] In statistical mechanics, temperature is the measure of the average kinetic energy stored in a molecule (also known as the thermal energy). The methods of storing this energy are dictated by the degrees of freedom of the molecule itself (energy modes). Thermal (kinetic) energy added to a gas or liquid (an endothermic process) produces translational, rotational, and vibrational motion. In contrast, a solid can only increase its internal energy by exciting additional vibrational modes, as the crystal lattice structure prevents both translational and rotational motion. These heated gas molecules have a greater speed range (wider distribution of speeds) with a higher average or mean speed. The variance of this distribution is due to the speeds of individual particles constantly varying, due to repeated collisions with other particles. The speed range can be described by the Maxwell–Boltzmann distribution. Use of this distribution implies ideal gases near thermodynamic equilibrium for the system of particles being considered.
Specific volume[edit]
The symbol used to represent specific volume in equations is «v» with SI units of cubic meters per kilogram.
The symbol used to represent volume in equations is «V» with SI units of cubic meters.
When performing a thermodynamic analysis, it is typical to speak of intensive and extensive properties. Properties which depend on the amount of gas (either by mass or volume) are called extensive properties, while properties that do not depend on the amount of gas are called intensive properties. Specific volume is an example of an intensive property because it is the ratio of volume occupied by a unit of mass of a gas that is identical throughout a system at equilibrium.[17] 1000 atoms a gas occupy the same space as any other 1000 atoms for any given temperature and pressure. This concept is easier to visualize for solids such as iron which are incompressible compared to gases. However, volume itself — not specific — is an extensive property.
Density[edit]
The symbol used to represent density in equations is ρ (rho) with SI units of kilograms per cubic meter. This term is the reciprocal of specific volume.
Since gas molecules can move freely within a container, their mass is normally characterized by density. Density is the amount of mass per unit volume of a substance, or the inverse of specific volume. For gases, the density can vary over a wide range because the particles are free to move closer together when constrained by pressure or volume. This variation of density is referred to as compressibility. Like pressure and temperature, density is a state variable of a gas and the change in density during any process is governed by the laws of thermodynamics. For a static gas, the density is the same throughout the entire container. Density is therefore a scalar quantity. It can be shown by kinetic theory that the density is inversely proportional to the size of the container in which a fixed mass of gas is confined. In this case of a fixed mass, the density decreases as the volume increases.
Microscopic view of gases[edit]
If one could observe a gas under a powerful microscope, one would see a collection of particles without any definite shape or volume that are in more or less random motion. These gas particles only change direction when they collide with another particle or with the sides of the container. This microscopic view of gas is well-described by statistical mechanics, but it can be described by many different theories. The kinetic theory of gases, which makes the assumption that these collisions are perfectly elastic, does not account for intermolecular forces of attraction and repulsion.
Kinetic theory of gases[edit]
Kinetic theory provides insight into the macroscopic properties of gases by considering their molecular composition and motion. Starting with the definitions of momentum and kinetic energy,[18] one can use the conservation of momentum and geometric relationships of a cube to relate macroscopic system properties of temperature and pressure to the microscopic property of kinetic energy per molecule. The theory provides averaged values for these two properties.
The kinetic theory of gases can help explain how the system (the collection of gas particles being considered) responds to changes in temperature, with a corresponding change in kinetic energy.
For example: Imagine you have a sealed container of a fixed-size (a constant volume), containing a fixed-number of gas particles; starting from absolute zero (the theoretical temperature at which atoms or molecules have no thermal energy, i.e. are not moving or vibrating), you begin to add energy to the system by heating the container, so that energy transfers to the particles inside. Once their internal energy is above zero-point energy, meaning their kinetic energy (also known as thermal energy) is non-zero, the gas particles will begin to move around the container. As the box is further heated (as more energy is added), the individual particles increase their average speed as the system’s total internal energy increases. The higher average-speed of all the particles leads to a greater rate at which collisions happen (i.e. greater number of collisions per unit of time), between particles and the container, as well as between the particles themselves.
The macroscopic, measurable quantity of pressure, is the direct result of these microscopic particle collisions with the surface, over which, individual molecules exert a small force, each contributing to the total force applied within a specific area. (Read «Pressure» in the above section «Macroscopic view of gases«.)
Likewise, the macroscopically measurable quantity of temperature, is a quantification of the overall amount of motion, or kinetic energy that the particles exhibit. (Read «Temperature» in the above section «Macroscopic view of gases«.)
Thermal motion and statistical mechanics[edit]
In the kinetic theory of gases, kinetic energy is assumed to purely consist of linear translations according to a speed distribution of particles in the system. However, in real gases and other real substances, the motions which define the kinetic energy of a system (which collectively determine the temperature), are much more complex than simple linear translation due to the more complex structure of molecules, compared to single atoms which act similarly to point-masses. In real thermodynamic systems, quantum phenomena play a large role in determining thermal motions. The random, thermal motions (kinetic energy) in molecules is a combination of a finite set of possible motions including translation, rotation, and vibration. This finite range of possible motions, along with the finite set of molecules in the system, leads to a finite number of microstates within the system; we call the set of all microstates an ensemble. Specific to atomic or molecular systems, we could potentially have three different kinds of ensemble, depending on the situation: microcanonical ensemble, canonical ensemble, or grand canonical ensemble. Specific combinations of microstates within an ensemble are how we truly define macrostate of the system (temperature, pressure, energy, etc.). In order to do that, we must first count all microstates though use of a partition function. The use of statistical mechanics and the partition function is an important tool throughout all of physical chemistry, because it is the key to connection between the microscopic states of a system and the macroscopic variables which we can measure, such as temperature, pressure, heat capacity, internal energy, enthalpy, and entropy, just to name a few. (Read: Partition function Meaning and significance)
Using the partition function to find the energy of a molecule, or system of molecules, can sometimes be approximated by the Equipartition theorem, which greatly-simplifies calculation. However, this method assumes all molecular degrees of freedom are equally populated, and therefore equally utilized for storing energy within the molecule. It would imply that internal energy changes linearly with temperature, which is not the case. This ignores the fact that heat capacity changes with temperature, due to certain degrees of freedom being unreachable (a.k.a. «frozen out») at lower temperatures. As internal energy of molecules increases, so does the ability to store energy within additional degrees of freedom. As more degrees of freedom become available to hold energy, this causes the molar heat capacity of the substance to increase.[19]
Random motion of gas particles results in diffusion.
Brownian motion[edit]
Brownian motion is the mathematical model used to describe the random movement of particles suspended in a fluid. The gas particle animation, using pink and green particles, illustrates how this behavior results in the spreading out of gases (entropy). These events are also described by particle theory.
Since it is at the limit of (or beyond) current technology to observe individual gas particles (atoms or molecules), only theoretical calculations give suggestions about how they move, but their motion is different from Brownian motion because Brownian motion involves a smooth drag due to the frictional force of many gas molecules, punctuated by violent collisions of an individual (or several) gas molecule(s) with the particle. The particle (generally consisting of millions or billions of atoms) thus moves in a jagged course, yet not so jagged as would be expected if an individual gas molecule were examined.
Intermolecular forces — the primary difference between Real and Ideal gases[edit]
Forces between two or more molecules or atoms, either attractive or repulsive, are called intermolecular forces. Intermolecular forces are experienced by molecules when they are within physical proximity of one another. These forces are very important for properly modeling molecular systems, as to accurately predict the microscopic behavior of molecules in any system, and therefore, are necessary for accurately predicting the physical properties of gases (and liquids) across wide variations in physical conditions.
Arising from the study of physical chemistry, one of the most prominent intermolecular forces throughout physics, are van der Waals forces. Van der Waals forces play a key role in determining nearly all physical properties of fluids such as viscosity, flow rate, and gas dynamics (see physical characteristics section). The van der Waals interactions between gas molecules, is the reason why modeling a «real gas» is more mathematically difficult than an «ideal gas». Ignoring these proximity-dependent forces allows a real gas to be treated like an ideal gas, which greatly simplifies calculation.
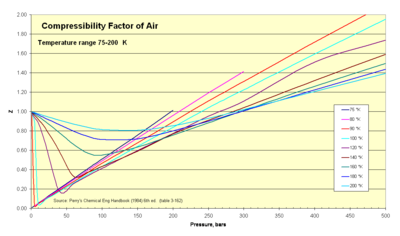
Isothermal curves depicting the non-ideality of a real gas. The changes in volume (depicted by Z, compressibility factor) which occur as the pressure is varied. The compressibility factor Z, is equal to the ratio Z = PV/nRT. An ideal gas, with compressibility factor Z = 1, is described by the horizontal line where the y-axis is equal to 1. Non-ideality can be described as the deviation of a gas above or below Z = 1.
The intermolecular attractions and repulsions between two gas molecules are dependent on the amount of distance between them. The combined attractions and repulsions are well-modelled by the Lennard-Jones potential, which is one of the most extensively studied of all interatomic potentials describing the potential energy of molecular systems. The Lennard-Jones potential between molecules can be broken down into two separate components: a long-distance attraction due to the London dispersion force, and a short-range repulsion due to electron-electron exchange interaction (which is related to the Pauli exclusion principle).
When two molecules are relatively distant (meaning they have a high potential energy), they experience a weak attracting force, causing them to move toward each other, lowering their potential energy. However, if the molecules are too far away, then they would not experience attractive force of any significance. Additionally, if the molecules get too close then they will collide, and experience a very high repulsive force (modelled by Hard spheres) which is a much stronger force than the attractions, so that any attraction due to proximity is disregarded.
As two molecules approach each other, from a distance that is neither too-far, nor too-close, their attraction increases as the magnitude of their potential energy increases (becoming more negative), and lowers their total internal energy.[20] The attraction causing the molecules to get closer, can only happen if the molecules remain in proximity for the duration of time it takes to physically move closer. Therefore, the attractive forces are strongest when the molecules move at low speeds. This means that the attraction between molecules is significant when gas temperatures is low. However, if you were to isothermally compress this cold gas into a small volume, forcing the molecules into close proximity, and raising the pressure, the repulsions will begin to dominate over the attractions, as the rate at which collisions are happening will increase significantly. Therefore, at low temperatures, and low pressures, attraction is the dominant intermolecular interaction.
If two molecules are moving at high speeds, in arbitrary directions, along non-intersecting paths, then they will not spend enough time in proximity to be affected by the attractive London-dispersion force. If the two molecules collide, they are moving too fast and their kinetic energy will be much greater than any attractive potential energy, so they will only experience repulsion upon colliding. Thus, attractions between molecules can be neglected at high temperatures due to high speeds. At high temperatures, and high pressures, repulsion is the dominant intermolecular interaction.
Accounting for the above stated effects which cause these attractions and repulsions, real gases, delineate from the ideal gas model by the following generalization:[21]
- At low temperatures, and low pressures, the volume occupied by a real gas, is less than the volume predicted by the ideal gas law.
- At high temperatures, and high pressures, the volume occupied by a real gas, is greater than the volume predicted by the ideal gas law.
Mathematical models[edit]
An equation of state (for gases) is a mathematical model used to roughly describe or predict the state properties of a gas. At present, there is no single equation of state that accurately predicts the properties of all gases under all conditions. Therefore, a number of much more accurate equations of state have been developed for gases in specific temperature and pressure ranges. The «gas models» that are most widely discussed are «perfect gas», «ideal gas» and «real gas». Each of these models has its own set of assumptions to facilitate the analysis of a given thermodynamic system.[22] Each successive model expands the temperature range of coverage to which it applies.
Ideal and perfect gas[edit]
The equation of state for an ideal or perfect gas is the ideal gas law and reads
where P is the pressure, V is the volume, n is amount of gas (in mol units), R is the universal gas constant, 8.314 J/(mol K), and T is the temperature. Written this way, it is sometimes called the «chemist’s version», since it emphasizes the number of molecules n. It can also be written as
where 
The ideal gas law does not make an assumption about the specific heat of a gas. In the most general case, the specific heat is a function of both temperature and pressure. If the pressure-dependence is neglected (and possibly the temperature-dependence as well) in a particular application, sometimes the gas is said to be a perfect gas, although the exact assumptions may vary depending on the author and/or field of science.
For an ideal gas, the ideal gas law applies without restrictions on the specific heat. An ideal gas is a simplified «real gas» with the assumption that the compressibility factor Z is set to 1 meaning that this pneumatic ratio remains constant. A compressibility factor of one also requires the four state variables to follow the ideal gas law.
This approximation is more suitable for applications in engineering although simpler models can be used to produce a «ball-park» range as to where the real solution should lie. An example where the «ideal gas approximation» would be suitable would be inside a combustion chamber of a jet engine.[23] It may also be useful to keep the elementary reactions and chemical dissociations for calculating emissions.
Real gas[edit]
21 April 1990 eruption of Mount Redoubt, Alaska, illustrating real gases not in thermodynamic equilibrium.
Each one of the assumptions listed below adds to the complexity of the problem’s solution. As the density of a gas increases with rising pressure, the intermolecular forces play a more substantial role in gas behavior which results in the ideal gas law no longer providing «reasonable» results. At the upper end of the engine temperature ranges (e.g. combustor sections – 1300 K), the complex fuel particles absorb internal energy by means of rotations and vibrations that cause their specific heats to vary from those of diatomic molecules and noble gases. At more than double that temperature, electronic excitation and dissociation of the gas particles begins to occur causing the pressure to adjust to a greater number of particles (transition from gas to plasma).[24] Finally, all of the thermodynamic processes were presumed to describe uniform gases whose velocities varied according to a fixed distribution. Using a non-equilibrium situation implies the flow field must be characterized in some manner to enable a solution. One of the first attempts to expand the boundaries of the ideal gas law was to include coverage for different thermodynamic processes by adjusting the equation to read pVn = constant and then varying the n through different values such as the specific heat ratio, γ.
Real gas effects include those adjustments made to account for a greater range of gas behavior:
- Compressibility effects (Z allowed to vary from 1.0)
- Variable heat capacity (specific heats vary with temperature)
- Van der Waals forces (related to compressibility, can substitute other equations of state)
- Non-equilibrium thermodynamic effects
- Issues with molecular dissociation and elementary reactions with variable composition.
For most applications, such a detailed analysis is excessive. Examples where real gas effects would have a significant impact would be on the Space Shuttle re-entry where extremely high temperatures and pressures were present or the gases produced during geological events as in the image of the 1990 eruption of Mount Redoubt.
Permanent gas[edit]
Permanent gas is a term used for a gas which has a critical temperature below the range of normal human-habitable temperatures and therefore cannot be liquefied by pressure within this range. Historically such gases were thought to be impossible to liquefy and would therefore permanently remain in the gaseous state. The term is relevant to ambient temperature storage and transport of gases at high pressure.[25]
Historical research[edit]
Boyle’s law[edit]
Boyle’s law was perhaps the first expression of an equation of state. In 1662 Robert Boyle performed a series of experiments employing a J-shaped glass tube, which was sealed on one end. Mercury was added to the tube, trapping a fixed quantity of air in the short, sealed end of the tube. Then the volume of gas was carefully measured as additional mercury was added to the tube. The pressure of the gas could be determined by the difference between the mercury level in the short end of the tube and that in the long, open end. The image of Boyle’s equipment shows some of the exotic tools used by Boyle during his study of gases.
Through these experiments, Boyle noted that the pressure exerted by a gas held at a constant temperature varies inversely with the volume of the gas.[26] For example, if the volume is halved, the pressure is doubled; and if the volume is doubled, the pressure is halved. Given the inverse relationship between pressure and volume, the product of pressure (P) and volume (V) is a constant (k) for a given mass of confined gas as long as the temperature is constant. Stated as a formula, thus is:
Because the before and after volumes and pressures of the fixed amount of gas, where the before and after temperatures are the same both equal the constant k, they can be related by the equation:

Charles’s law[edit]
In 1787, the French physicist and balloon pioneer, Jacques Charles, found that oxygen, nitrogen, hydrogen, carbon dioxide, and air expand to the same extent over the same 80 kelvin interval. He noted that, for an ideal gas at constant pressure, the volume is directly proportional to its temperature:
Gay-Lussac’s law[edit]
In 1802, Joseph Louis Gay-Lussac published results of similar, though more extensive experiments.[27] Gay-Lussac credited Charles’ earlier work by naming the law in his honor. Gay-Lussac himself is credited with the law describing pressure, which he found in 1809. It states that the pressure exerted on a container’s sides by an ideal gas is proportional to its temperature.
Avogadro’s law[edit]
In 1811, Amedeo Avogadro verified that equal volumes of pure gases contain the same number of particles. His theory was not generally accepted until 1858 when another Italian chemist Stanislao Cannizzaro was able to explain non-ideal exceptions. For his work with gases a century prior, the physical constant that bears his name (the Avogadro constant) is the number of atoms per mole of elemental carbon-12 (6.022×1023 mol−1). This specific number of gas particles, at standard temperature and pressure (ideal gas law) occupies 22.40 liters, which is referred to as the molar volume.
Avogadro’s law states that the volume occupied by an ideal gas is proportional to the amount of substance in the volume. This gives rise to the molar volume of a gas, which at STP is 22.4 dm3/mol (liters per mole). The relation is given by
where n is the amount of substance of gas (the number of molecules divided by the Avogadro constant).
Dalton’s law[edit]
In 1801, John Dalton published the law of partial pressures from his work with ideal gas law relationship: The pressure of a mixture of non reactive gases is equal to the sum of the pressures of all of the constituent gases alone. Mathematically, this can be represented for n species as:
- Pressuretotal = Pressure1 + Pressure2 + … + Pressuren
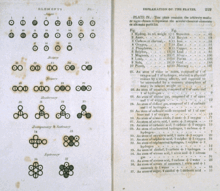
The image of Dalton’s journal depicts symbology he used as shorthand to record the path he followed. Among his key journal observations upon mixing unreactive «elastic fluids» (gases) were the following:[28]
- Unlike liquids, heavier gases did not drift to the bottom upon mixing.
- Gas particle identity played no role in determining final pressure (they behaved as if their size was negligible).
Special topics[edit]
Compressibility[edit]
Compressibility factors for air.
Thermodynamicists use this factor (Z) to alter the ideal gas equation to account for compressibility effects of real gases. This factor represents the ratio of actual to ideal specific volumes. It is sometimes referred to as a «fudge-factor» or correction to expand the useful range of the ideal gas law for design purposes. Usually this Z value is very close to unity. The compressibility factor image illustrates how Z varies over a range of very cold temperatures.
Reynolds number[edit]
In fluid mechanics, the Reynolds number is the ratio of inertial forces (vsρ) to viscous forces (μ/L). It is one of the most important dimensionless numbers in fluid dynamics and is used, usually along with other dimensionless numbers, to provide a criterion for determining dynamic similitude. As such, the Reynolds number provides the link between modeling results (design) and the full-scale actual conditions. It can also be used to characterize the flow.
Viscosity[edit]
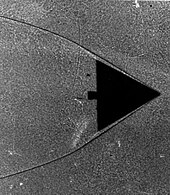
Viscosity, a physical property, is a measure of how well adjacent molecules stick to one another. A solid can withstand a shearing force due to the strength of these sticky intermolecular forces. A fluid will continuously deform when subjected to a similar load. While a gas has a lower value of viscosity than a liquid, it is still an observable property. If gases had no viscosity, then they would not stick to the surface of a wing and form a boundary layer. A study of the delta wing in the Schlieren image reveals that the gas particles stick to one another (see Boundary layer section).
Turbulence[edit]
Delta wing in wind tunnel. The shadows form as the indices of refraction change within the gas as it compresses on the leading edge of this wing.
In fluid dynamics, turbulence or turbulent flow is a flow regime characterized by chaotic, stochastic property changes. This includes low momentum diffusion, high momentum convection, and rapid variation of pressure and velocity in space and time. The satellite view of weather around Robinson Crusoe Islands illustrates one example.
Boundary layer[edit]
Particles will, in effect, «stick» to the surface of an object moving through it. This layer of particles is called the boundary layer. At the surface of the object, it is essentially static due to the friction of the surface. The object, with its boundary layer is effectively the new shape of the object that the rest of the molecules «see» as the object approaches. This boundary layer can separate from the surface, essentially creating a new surface and completely changing the flow path. The classical example of this is a stalling airfoil. The delta wing image clearly shows the boundary layer thickening as the gas flows from right to left along the leading edge.
Maximum entropy principle[edit]
As the total number of degrees of freedom approaches infinity, the system will be found in the macrostate that corresponds to the highest multiplicity. In order to illustrate this principle, observe the skin temperature of a frozen metal bar. Using a thermal image of the skin temperature, note the temperature distribution on the surface. This initial observation of temperature represents a «microstate». At some future time, a second observation of the skin temperature produces a second microstate. By continuing this observation process, it is possible to produce a series of microstates that illustrate the thermal history of the bar’s surface. Characterization of this historical series of microstates is possible by choosing the macrostate that successfully classifies them all into a single grouping.
Thermodynamic equilibrium[edit]
When energy transfer ceases from a system, this condition is referred to as thermodynamic equilibrium. Usually, this condition implies the system and surroundings are at the same temperature so that heat no longer transfers between them. It also implies that external forces are balanced (volume does not change), and all chemical reactions within the system are complete. The timeline varies for these events depending on the system in question. A container of ice allowed to melt at room temperature takes hours, while in semiconductors the heat transfer that occurs in the device transition from an on to off state could be on the order of a few nanoseconds.
See also[edit]
- Greenhouse gas
- List of gases
- Natural gas
- Volcanic gas
- Breathing gas
- Wind
Notes[edit]
- ^ «Gas». Merriam-Webster.
{{cite web}}: CS1 maint: url-status (link) - ^ This early 20th century discussion infers what is regarded as the plasma state. See page 137 of American Chemical Society, Faraday Society, Chemical Society (Great Britain) The Journal of Physical Chemistry, Volume 11 Cornell (1907).
- ^ Zelevinsky, Tanya (2009-11-09). «—just right for forming a Bose-Einstein condensate». Physics. 2 (20): 94. arXiv:0910.0634. doi:10.1103/PhysRevLett.103.200401. PMID 20365964. S2CID 14321276.
- ^ «Quantum Gas Microscope Offers Glimpse Of Quirky Ultracold Atoms». ScienceDaily. Retrieved 2023-02-06.
- ^ Helmont, Jan Baptist Van (1652). Ortus medicinae, id est initia physicae inaudita… authore Joanne Baptista Van Helmont,… (in Latin). apud L. Elzevirium.
- ^ Ley, Willy (June 1966). «The Re-Designed Solar System». For Your Information. Galaxy Science Fiction. pp. 94–106.
- ^ Harper, Douglas. «gas». Online Etymology Dictionary.
- ^ Draper, John William (1861). A textbook on chemistry. New York: Harper and Sons. p. 178.
- ^ ««gas, n.1 and adj.»«. OED Online. Oxford University Press. June 2021.
There is probably no foundation in the idea (found from the 18th cent. onwards, e.g. in J. Priestley On Air (1774) Introd. 3) that van Helmont modelled gas on Dutch geest spirit, or any of its cognates
- ^ Barzun, Jacques (2000). For Dawn to Decadence: 500 Years of Western Cultural Life. New York: HarperCollins Publishers. p. 199.
- ^ The authors make the connection between molecular forces of metals and their corresponding physical properties. By extension, this concept would apply to gases as well, though not universally. Cornell (1907) pp. 164–5.
- ^ One noticeable exception to this physical property connection is conductivity which varies depending on the state of matter (ionic compounds in water) as described by Michael Faraday in 1833 when he noted that ice does not conduct a current. See page 45 of John Tyndall’s Faraday as a Discoverer (1868).
- ^ John S. Hutchinson (2008). Concept Development Studies in Chemistry. p. 67.
- ^ Anderson, p.501
- ^ J. Clerk Maxwell (1904). Theory of Heat. Mineola: Dover Publications. pp. 319–20. ISBN 978-0-486-41735-6.
- ^ See pages 137–8 of Society, Cornell (1907).
- ^ Kenneth Wark (1977). Thermodynamics (3 ed.). McGraw-Hill. p. 12. ISBN 978-0-07-068280-1.
- ^ For assumptions of kinetic theory see McPherson, pp.60–61
- ^ Jeschke, Gunnar (26 November 2020). «Canonical Ensemble». Archived from the original on 2021-05-20.
- ^ «Lennard-Jones Potential — Chemistry LibreTexts». 2020-08-22. Archived from the original on 2020-08-22. Retrieved 2021-05-20.
- ^ «14.11: Real and Ideal Gases — Chemistry LibreTexts». 2021-02-06. Archived from the original on 2021-02-06. Retrieved 2021-05-20.
- ^ Anderson, pp. 289–291
- ^ John, p.205
- ^ John, pp. 247–56
- ^ «Permanent gas». www.oxfordreference.com. Oxford University Press. Retrieved 3 April 2021.
- ^ McPherson, pp.52–55
- ^ McPherson, pp.55–60
- ^ John P. Millington (1906). John Dalton. pp. 72, 77–78.
References[edit]
- Anderson, John D. (1984). Fundamentals of Aerodynamics. McGraw-Hill Higher Education. ISBN 978-0-07-001656-9.
- John, James (1984). Gas Dynamics. Allyn and Bacon. ISBN 978-0-205-08014-4.
- McPherson, William; Henderson, William (1917). An Elementary study of chemistry.
Further reading[edit]
Look up gas in Wiktionary, the free dictionary.
Wikimedia Commons has media related to Gases.
- Philip Hill and Carl Peterson. Mechanics and Thermodynamics of Propulsion: Second Edition Addison-Wesley, 1992. ISBN 0-201-14659-2
- National Aeronautics and Space Administration (NASA). Animated Gas Lab. Accessed February 2008.
- Georgia State University. HyperPhysics. Accessed February 2008.
- Antony Lewis WordWeb. Accessed February 2008.
- Northwestern Michigan College The Gaseous State. Accessed February 2008.
- Lewes, Vivian Byam; Lunge, Georg (1911). «Gas» . Encyclopædia Britannica. Vol. 11 (11th ed.). p. 481–493.
Noun
Carbon monoxide is a poisonous gas.
We heat our house with gas.
Do you have a gas stove or an electric one?
The car gets good gas mileage.
The car almost ran out of gas.
He was driving with one foot on the gas and one foot on the brake.
Verb
soldiers gassed on the battlefield
We stopped to gas the car.
See More
Recent Examples on the Web
The work is being paid for with money received through the Gulf of Mexico Energy and Security Act (GOMESA), which allocates revenue from offshore oil and gas leases.
—
President Biden’s withdrawal of remaining Beaufort Sea territory from future oil and gas leasing schedules.
—
In their annual survey of companies representing about 70% of global output, the analysts estimate that these companies will spend $376 billion finding and extracting oil and gas this year.
—
Understand the Latest News on Climate Change Card 1 of 6 Oil and gas projects.
—
In 2021, 58 workers died in the oil and gas industries, according US Bureau of Labor Statistics.
—
In the Cuvette-Centrale basin in Congo, a dense and ecologically thriving forest that’s home to the largest population of lowland gorillas, sections of the peatlands — the continent’s largest — went up for oil and gas auction last year.
—
Evidence abounds that the effort to isolate Putin has failed and that sanctions haven’t stopped Russia, thanks to its oil and gas exports.
—
That 2022 output was generated by 24% fewer workers than a decade earlier, and the state had a record haul from oil and gas production taxes.
—
The good news is that proper ventilation will help the off-gassing to occur quickly, so any odors should dissipate quickly.
—
To me, some of the biggest issues of the Texans defenses in recent years is staying on the field too long and getting gassed because the offense could not help them at all.
—
Next came a debacle during last year’s Champions League Cup final when British soccer fans were gassed by police at the Stade de France.
—
There’s even a charge port for gassing up your mobile tech.
—
Get your car gassed up or charged up, bring your cellphone to purchase a ticket on GoFan.co and prepare to enjoy the thrill of victory or the agony of defeat in the sports of basketball, soccer and wrestling.
—
If people gas you up, your head will get too big.
—
While other people might use fake humility to appear down-to-earth, the TikToker doesn’t mind coming off a little cocky to the public — after all, she’s had to gas herself up to make it this far.
—
But the rest had to gas up their cars and join the long lines snaking on roads toward the borders.
—
See More
These examples are programmatically compiled from various online sources to illustrate current usage of the word ‘gas.’ Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
-
Defenition of the word gas
- A substance that continues to occupy in a continuous manner the whole of the space in which it is placed, however large or small this place is made, the temperature remaining constant.
- A fuel for internal combustion engines consisting essentially of volatile flammable liquid hydrocarbons derived from crude petroleum.
- To show off.
- a pedal that controls the throttle valve; «he stepped on the gas»
- show off
- a state of excessive gas in the alimentary canal
- the state of matter distinguished from the solid and liquid states by: relatively low density and viscosity; relatively great expansion and contraction with changes in pressure and temperature; the ability to diffuse readily; and the spontaneous tendency to become distributed uniformly throughout any container
- a fluid in the gaseous state having neither independent shape nor volume and being able to expand indefinitely
- attack with gas; subject to gas fumes; «The despot gassed the rebellious tribes»
- a volatile flammable mixture of hydrocarbons (hexane and heptane and octane etc.) derived from petroleum; used mainly as a fuel in internal-combustion engines
- a fossil fuel in the gaseous state; used for cooking and heating homes
- the state of matter distinguished from the solid and liquid states by: relatively low density and viscosity; relatively great expansion and contraction with changes in pressure and temperature; the ability to diffuse readily; and the spontaneous tendency
- a pedal that controls the throttle valve
- attack with gas; subject to gas fumes
Synonyms for the word gas
-
- accelerator
- accelerator pedal
- blow
- bluster
- boast
- brag
- chat
- chatter
- flatulence
- flatulency
- gab
- gas pedal
- gasconade
- gasolene
- gasoline
- gun
- natural gas
- petrol
- shoot a line
- swap gossip
- swash
- throttle
- tout
- vaunt
Similar words in the gas
-
- gas
- gascony
- gascony’s
- gaseous
- gases
- gash
- gashed
- gashes
- gashing
- gasket
- gasket’s
- gaskets
- gaslight
- gaslight’s
- gaslights
- gasohol
- gasoline
- gasped
- gasping
- gasps
- gassed
- gasser
- gasser’s
- gassier
- gassiest
- gassing
- gassy
- gastric
- gastritis
- gastritis’s
- gastronomic
- gastronomical
- gastronomy
- gastronomy’s
- gasworks
- gasworks’s
Meronymys for the word gas
-
- aeroplane
- airplane
- auto
- automobile
- car
- machine
- methane
- motorcar
- plane
Hyponyms for the word gas
-
- afterdamp
- air
- air gas
- argonon
- arsine
- atmosphere
- atomic number 1
- atomic number 17
- atomic number 7
- atomic number 8
- atomic number 9
- blow gas
- blowing gas
- bottled gas
- butane
- butene
- butylene
- chlorine
- Cl
- compressed gas
- crow
- cyanogen
- ethene
- ethylene
- exhaust
- exhaust fumes
- F
- firedamp
- fluorine
- formaldehyde
- fumes
- gloat
- greenhouse emission
- greenhouse gas
- H
- hydrogen
- ideal gas
- inert gas
- inhalant
- lachrymator
- lacrimator
- leaded gasoline
- leaded petrol
- liquefied petroleum gas
- mephitis
- methanal
- methane
- N
- napalm
- nitric oxide
- nitrogen
- nitrogen dioxide
- noble gas
- O
- oxygen
- ozone
- perfect gas
- phosgene
- phosphine
- poison gas
- producer gas
- propane
- propene
- propylene
- puff
- sewer gas
- sublimate
- sulfur dioxide
- sulphur dioxide
- tear gas
- teargas
- triumph
- unleaded gasoline
- unleaded petrol
- water gas
Hypernyms for the word gas
-
- amplify
- assail
- attack
- combustible
- combustible material
- exaggerate
- fluid
- foot lever
- foot pedal
- fossil fuel
- fuel
- hydrocarbon
- hyerbolise
- hyperbolise
- hyperbolize
- magnify
- overdraw
- overstate
- pedal
- physical condition
- physiological condition
- physiological state
- state
- state of matter
- treadle
See other words
-
- What is archiv
- The definition of architektur
- The interpretation of the word elternteil
- What is meant by fink
- The lexical meaning eltern
- The dictionary meaning of the word gambier
- The grammatical meaning of the word architektin
- Meaning of the word fingernagel
- Literal and figurative meaning of the word eloquenz
- The origin of the word flachwichser
- Synonym for the word endemie
- Antonyms for the word aristokrat
- Homonyms for the word flakon
- Hyponyms for the word chronometer
- Holonyms for the word energie
- Hypernyms for the word flasche
- Proverbs and sayings for the word aristokratin
- Translation of the word in other languages arithmetik
English[edit]
Pronunciation[edit]
- enPR: găs, IPA(key): /ɡæs/
- enPR: găz, IPA(key): /ɡæz/(regional)
- Rhymes: -æs
- Rhymes: -æz(some speakers)
Etymology 1[edit]
Borrowed from Dutch gas, coined by chemist Jan Baptist van Helmont in Ortus Medicinae. Derived from Ancient Greek χάος (kháos, “chasm, void, empty space”); perhaps also inspired by geest (“breath, vapour, spirit”). Doublet of chaos. First attested in 1648.
Noun[edit]
gas (countable and uncountable, plural gases or gasses)
- (uncountable, chemistry) Matter in an intermediate state between liquid and plasma that can be contained only if it is fully surrounded by a solid (or in a bubble of liquid, or held together by gravitational pull); it can condense into a liquid, or can (rarely) become a solid directly by deposition.
-
2013 July–August, Lee S. Langston, “The Adaptable Gas Turbine”, in American Scientist[1]:
-
Turbines have been around for a long time—windmills and water wheels are early examples. The name comes from the Latin turbo, meaning vortex, and thus the defining property of a turbine is that a fluid or gas turns the blades of a rotor, which is attached to a shaft that can perform useful work.
-
-
A lot of gas had escaped from the cylinder.
- Synonyms: vapor, vapour
- (uncountable) A flammable gaseous hydrocarbon or hydrocarbon mixture used as a fuel, e.g. for cooking, heating, electricity generation or as a fuel in internal combustion engines in vehicles, especially natural gas.
-
Gas-fired power stations have largely replaced coal-burning ones.
-
- (uncountable, military) Poison gas.
-
The artillery fired gas shells into the enemy trenches.
-
-
- (countable, chemistry) A chemical element or compound in such a state.
-
The atmosphere is made up of a number of different gases.
-
- (countable) A hob on a gas cooker.
-
She turned the gas on, put the potatoes on, then lit the oven.
-
- (uncountable) Methane or other waste gases trapped in one’s belly as a result of the digestive process; flatus.
- Synonym: wind
-
My tummy hurts so bad – I have gas.
-
2008, Nicholas Drayson, A Guide to the Birds of East Africa, page 72:
-
But anyone with that many large brown birds aroost in his cranium and that much gas in his bottom was clearly not a well person.
-
- (slang) A humorous or entertaining event or person.
-
1963 May, Gloria Steinem, “A Bunny’s Tale”, in Show Magazine[2], archived from the original on 2017-10-04:
-
Two more girls came in, one in bright pink stretch pants and the other in purple. “Man this place is a gas,” said pink.
-
-
1971, Marc Bolan (lyrics and music), “Life’s a Gas”, in Electric Warrior, performed by T. Rex:
-
No it really doesn’t matter at all / Life’s a gas / I hope it’s going to last
-
-
1978, “Heart of Glass”, in Parallel Lines, performed by Blondie:
-
Once I had a love and it was a gas / Soon turned out had a heart of glass
-
-
1979, “Belsen Was a Gas”, in The Great Rock ‘n’ Roll Swindle, performed by Sex Pistols:
-
Be a man, Be a man / Belsen was a gas / Be a man, kill someone
-
-
2011 October 11, “Jumping Jack Flash (Live 1973)”, in Brussels Affair (Live 1973)[3], performed by The Rolling Stones:
-
One two! I was born in a cross-fire hurricane. And I howled at the maw in the drivin’ rain. But it’s all right now, in fact, it’s a gas. But it’s all right. I’m Jumpin’ Jack Flash. It’s a gas, gas, gas.
-
-
- (slang) Frothy or boastful talk; chatter.
- (baseball) A fastball.
-
The closer threw him nothing but gas.
-
- (medicine, colloquial) Arterial or venous blood gas.
- (slang, uncountable) Marijuana, typically of high quality.
Derived terms[edit]
- air gas
- base gas
- bottled gas
- breathing gas
- Calor gas
- camping gas
- carbonic acid gas
- carrier gas
- CN gas
- coal gas
- coal seam gas (CSG)
- coke oven gas
- combined gas law
- cooking on gas
- cooking with gas
- CS gas
- degas
- drip gas
- Fermi gas
- filling gas
- flue gas
- freedom gas
- gas and air
- gas and gaiters
- gas bar
- gas bladder
- gas boat
- gas bottle
- gas bracket
- gas burner
- gas centrifuge
- gas chamber
- gas check
- gas chromatograph
- gas chromatography
- gas clathrate
- gas coal
- gas constant
- gas cylinder
- gas dynamics
- gas engine
- gas field
- gas fire
- gas fitter
- gas flaring
- gas gauge
- gas generator
- gas giant
- gas hydrate
- gas jar
- gas jet
- gas jockey
- gas line
- gas main
- gas mantle
- gas mark
- gas mask
- gas mechanics
- gas meter
- gas meter bandit
- gas passer
- gas pipeline
- gas plant
- gas poker
- gas syringe
- gas tar
- gas turbine
- gas van
- gas washer
- gas water
- gas-fired
- gas-liquid chromatography
- gas-masked
- gas-powered
- gasbag
- gaseous
- gasholder
- gasify
- gaslamp, gas lamp
- gasless
- gaslight, gas light, gas-light
- gaslike
- gaslit, gas-lit
- gasman
- gasometer
- gassy
- gasworks, gas works
- greenhouse gas
- happy gas
- have a gas
- hydrogen gas
- hydrogen gas electrode
- ideal gas
- ideal gas law
- illuminating gas
- inert gas
- inert gas narcosis
- Knudsen gas
- laughing gas
- lifting gas
- liquefied natural gas
- liquefied petroleum gas (LPG)
- liquid gas
- liquid natural gas
- liquified natural gas
- marsh gas
- mustard gas
- natural gas
- natural gas liquid
- nerve gas
- Nevada gas
- noble gas
- OC gas
- off-gas
- oil gas
- olefiant gas
- outgas
- oxygen gas
- packaging gas
- pass gas
- pepper gas, pepper-gas
- Pintsch gas
- poison gas
- power to gas
- producer gas
- purple gas
- rotten egg gas, rotten-egg gas
- sewer gas
- shale gas
- sour gas
- super greenhouse gas
- swamp gas
- synthesis gas, syngas
- take gas
- tear gas, tear-gas
- town gas
- towns gas
- ullage gas
- van der Waals gas
- water gas
- white gas
Translations[edit]
(uncountable, chemistry) state of matter
- Afrikaans: gas (af)
- Albanian: gaz (sq) m
- Arabic: غَاز m (ḡāz)
- Armenian: գազ (hy) (gaz)
- Assamese: গেছ (ges)
- Azerbaijani: qaz (az)
- Basque: gas (eu)
- Belarusian: газ m (haz)
- Bengali: গ্যাস (gêś)
- Bulgarian: газ (bg) m (gaz)
- Burmese: ဓာတ်ငွေ့ (my) (dhatngwe.)
- Catalan: gas (ca) m
- Chinese:
- Cantonese: 氣體/气体 (hei3 tai2)
- Mandarin: 氣體/气体 (zh) (qìtǐ), 氣/气 (zh) (qì)
- Min Nan: 氣體/气体 (zh-min-nan) (khì-thé)
- Czech: plyn (cs) m
- Danish: gas (da) n
- Dutch: gas (nl) n
- Erzya: лекс (ľeks)
- Esperanto: gaso (eo)
- Estonian: gaas
- Faroese: gass n
- Finnish: kaasu (fi)
- French: gaz (fr) m sg or m pl
- Galician: gas (gl) m
- Georgian: გაზი (gazi), აირი (ka) (airi)
- German: Gas (de) n
- Greek: αέριο (el) n (aério), ατμός (el) m (atmós)
- Greenlandic: gassi
- Gujarati: વાયુ (gu) m (vāyu)
- Hebrew: גַּז (he) m (gaz)
- Hindi: गैस (hi) f (gais)
- Hungarian: gáz (hu)
- Icelandic: gas (is) n
- Ido: gaso (io)
- Indonesian: gas (id)
- Interlingua: gas
- Irish: gás m
- Italian: gas (it) m
- Japanese: 気体 (ja) (きたい, kitai), ガス (ja) (gasu)
- Kannada: ಅನಿಲ (kn) (anila)
- Kazakh: газ (kk) (gaz)
- Khmer: ឧស្ម័ន (ŭsmăn), ហ្គាស (hkas)
- Korean: 가스 (ko) (gaseu), 기체(氣體) (ko) (giche)
- Kurdish:
- Northern Kurdish: gaz (ku)
- Kyrgyz: газ (ky) (gaz)
- Lao: ອາຍໄຕ້ (ʼāi tai), ອາຍ (ʼāi), ແກັສ (kæt)
- Latin: gas (la) n, gasum n, gasium (la) n
- Latvian: gāze f
- Lithuanian: dujos f pl
- Macedonian: гас m (gas)
- Malay: gas (ms)
- Maltese: gass m
- Maori: haurehu, kāhi
- Marathi: वायू (vāyū)
- Mongolian:
- Cyrillic: хий (mn) (xii)
- Norwegian:
- Bokmål: gass (no) m
- Nynorsk: gass m
- Oriya: ଗ୍ୟାସ୍ (or) (gyas)
- Pashto: ګاز m (gāz), غاز (ps) (ǧāz)
- Persian: گاز (fa) (gâz)
- Polish: gaz (pl) m
- Portuguese: gás (pt) m
- Punjabi: ਗੈਸ ? (gais), ਫੂ ? (phū)
- Romagnol: gas m
- Romanian: gaz (ro) n, gaze n pl
- Russian: газ (ru) m (gaz)
- Scottish Gaelic: gas (gd) m
- Serbo-Croatian:
- Cyrillic: пли̑н m, га̑с m
- Roman: plȋn (sh) m, gȃs (sh) m
- Sicilian: gas m
- Silesian: goz m
- Sinhalese: වායු (wāyu)
- Slovak: plyn m
- Slovene: plin (sl) m
- Spanish: gas (es) m
- Swahili: gesi (sw) class 9/10
- Swedish: gas (sv) c
- Tagalog: buhag
- Tajik: газ (tg) (gaz)
- Tamil: வளிமம் (ta) (vaḷimam), வாயு (ta) (vāyu)
- Tatar: газ (ğaz)
- Telugu: వాయువు (te) (vāyuvu)
- Thai: ก๊าซ (th) (gáat), แก๊ส (th) (gɛ́ɛt)
- Tibetan: རླངས་རླུང (rlangs rlung)
- Turkish: gaz (tr)
- Turkmen: gaz
- Ukrainian: газ (uk) m (haz)
- Urdu: گیس f (gais), فارغہ ?
- Uyghur: گاز (gaz)
- Uzbek: gaz (uz)
- Vietnamese: khí tê, khí (vi), chất khí (vi)
- Volapük: vap (vo)
- Welsh: nwy (cy) m
- West Frisian: gas n
- Yiddish: גאַז n (gaz)
(US) gas in digestion
- Belarusian: га́зы m pl (házy)
- Bulgarian: га́зове (bg) m pl (gázove)
- Finnish: ilmavaiva (fi), ilmavaivat (fi)
- French: gaz (fr) m sg or m pl
- Georgian: გაზები (gazebi)
- German: Blähung (de) f
- Greek: αέριο (el) n (aério)
- Hawaiian: hūea
- Hebrew: גזים m pl (gázim)
- Hungarian: szél (hu), bélgáz (hu)
- Italian: gas (it) m
- Japanese: ガス (ja) (gasu)
- Korean: 가스 (ko) (gaseu)
- Latvian: gāze f, gāzes f pl
- Macedonian: гасови m pl (gasovi)
- Polish: gazy m pl
- Portuguese: gases (pt) m pl
- Romanian: gaze n pl
- Russian: га́зы (ru) m pl (gázy)
- Sicilian: aria (scn) f
- Slovak: plyny m pl
- Swedish: gaser (sv) c pl
- Turkish: gaz (tr)
- Ukrainian: га́зи (uk) m pl (házy)
See also[edit]
- fluid
- liquid
- solid
Verb[edit]
gas (third-person singular simple present gases or gasses, present participle gassing, simple past and past participle gassed)
- (transitive) To attack or kill with poison gas.
-
The Nazis gassed millions of Jews during the Holocaust.
-
He never fully recovered after he was gassed on the Western Front.
-
- (intransitive, slang) To talk in a boastful or vapid way; chatter.
-
1899, Stephen Crane, chapter 1, in Twelve O’Clock:
-
[…] (it was the town’s humour to be always gassing of phantom investors who were likely to come any moment and pay a thousand prices for everything) — “ […] Them rich fellers, they don’t make no bad breaks with their money. […] ”
-
- 1955, C. S. Lewis, The Magician’s Nephew, Collins, 1998, Chapter 3,
- «Well don’t keep on gassing about it,» said Digory.
-
- (transitive, slang) To impose upon by talking boastfully.
-
2018 September 14, “Don’t Gas Me”, in Don’t Gas Me[4], performed by Dizzy Rascal:
-
I went shop and the boss man said «Don’t pay me it’s fine» and I said …(whaaat): «You ain’t gotta gas, I’m gas fam» ( don’t gas me), «You ain’t gotta gas, I’m gas fam».
-
-
- (intransitive) To emit gas.
-
The battery cell was gassing.
-
- (transitive) To impregnate with gas.
-
to gas lime with chlorine in the manufacture of bleaching powder
-
- (transitive) To singe, as in a gas flame, so as to remove loose fibers.
-
to gas thread
-
Translations[edit]
to kill with poisonous gas
Etymology 2[edit]
Clipping of gasoline.
Noun[edit]
gas (countable and uncountable, plural gases or gasses)
- (uncountable, Canada, US) Gasoline, a light derivative of petroleum used as fuel.
- Synonyms: (US) gasoline, (British) petrol, see also Thesaurus:petroleum
- (uncountable, Canada, US, by extension) Ellipsis of gas pedal.
- (uncountable, cryptocurrencies) An internal virtual currency used in Ethereum to pay for certain operations, such as blockchain transactions.
- Coordinate term: Ether
- gas fee
-
2018, Andreas M. Antonopoulos; Gavin Wood, Mastering Ethereum: Building Smart Contracts and DApps[5], O’Reilly Media, →ISBN:
-
Gas is the fuel of Ethereum. Gas is not ether–it’s a separate virtual currency with its own exchange rate against ether. Ethereum uses gas to control the amount of resources that transactions can use […]
-
-
2021 November 6, Ben Butler, “Australian banks are opening up to cryptocurrency: what does it mean for you?”, in The Guardian[6]:
-
The average “gas fee” – transaction cost – of an Ethereum transaction is between US$85 and US $156, according to crypto.com data.
-
Derived terms[edit]
- autogas
- avgas, av-gas
- gas generator
- gas oil
- gas out
- gas pedal
- gas pump
- gas station
- gas tank
- gas truck
- gas up
- gas-guzzler, gas guzzler
- gas-guzzling, gas guzzling
- gas-hog
- gasless
- gasohol
- hit the gas
- mogas
- out of gas
- run out of gas
- step on the gas
- throw gas on the fire
Translations[edit]
fuel
- Armenian: բենզին (hy) (benzin)
- Bulgarian: бензин (bg) m (benzin)
- Chinese:
- Mandarin: (please verify) 汽油 (zh) (qìyóu)
- Czech: benzín (cs) m
- Dutch: benzine (nl) f
- Finnish: bensiini (fi), bensa (fi)
- French: essence (fr) f
- Georgian: საწვავი (sac̣vavi), ბენზინი (benzini)
- German: Benzin (de) n
- Greek: βενζίνη (el) f (venzíni)
- Hebrew: דלק (he) m (délek)
- Icelandic: bensín (is) n
- Interlingua: gasolina
- Italian: benzina (it) f
- Japanese: ガソリン (ja) (がそりん, gasorin)
- Korean: 기름 (ko) (gireum)
- Macedonian: бензин m (benzin)
- Polish: benzyna (pl) f
- Portuguese: gasolina (pt) f
- Romanian: benzină (ro)
- Russian: бензи́н (ru) m (benzín)
- Sicilian: binzina (scn), nàffita f, gas m, carburanti m
- Slovak: benzín m
- Spanish: gasolina (es), bencina (es), nafta (es)
- Swedish: bensin (sv)
- Tok Pisin: bensin (tpi)
- Volapük: bänsin (vo)
Verb[edit]
gas (third-person singular simple present gases or gasses, present participle gassing, simple past and past participle gassed)
- (US) To give a vehicle more fuel in order to accelerate it.
-
The cops are coming. Gas it!
- Synonyms: hit the gas, step on the gas
-
- (US) To fill (a vehicle’s fuel tank) with fuel.
- Synonym: refuel
Derived terms[edit]
- gas and dash, gas-and-dash
- gas and go, gas-and-go
- gas up
Translations[edit]
fill a vehicle’s fuel tank
- Bulgarian: зареждам (bg) (zareždam)
- Dutch: tanken (nl)
- Georgian: საწვავით გამართვა (sac̣vavit gamartva)
- German: auftanken (de), tanken (de)
- Portuguese: completar (pt)
- Russian: заправля́ть (ru) impf (zapravljátʹ), запра́вить (ru) pf (zaprávitʹ)
- Spanish: echar gasolina, llenar el tanque, repostar (es)
Etymology 3[edit]
Compare the slang usage of «a gas», above.
Adjective[edit]
gas (comparative gasser, superlative gassest)
- (slang) Comical, zany; fun, amusing.
-
2016, Liz Nugent, Lying In Wait, →ISBN, page 113:
-
The other models were gas fun, though they were all a bit hoity-toity.
-
-
2018 September 14, “Don’t Gas Me”, in Don’t Gas Me[7], performed by Dizzy Rascal:
-
I went shop and the boss man said «Don’t pay me it’s fine» and I said …(whaaat): «You ain’t gotta gas, I’m gas fam» ( don’t gas me), «You ain’t gotta gas, I’m gas fam».
-
-
Mary’s new boyfriend is a gas man.
-
It was gas when the bird flew into the classroom.
-
Anagrams[edit]
- AGS, AGs, Ags., GSA, SAG, SGA, Sag, sag
Afrikaans[edit]
Etymology 1[edit]
From Dutch gast.
Noun[edit]
gas (plural gaste)
- guest
Etymology 2[edit]
From Dutch gas.
Noun[edit]
gas (plural gasse)
- gas (substance in gaseous phase)
Basque[edit]
Noun[edit]
gas inan
- gas
Declension[edit]
Declension of gas (inanimate, ending in consonant)
Derived terms[edit]
- gaseoso
Catalan[edit]
Pronunciation[edit]
- (Balearic, Central, Valencian) IPA(key): /ˈɡas/
Noun[edit]
gas m (plural gasos)
- gas
Derived terms[edit]
- biogàs
- cambra de gas
- gas d’envasat
- gas lacrimògen
- gas natural
- gas noble
- gasós
[edit]
- gasificar
- gasolina
Further reading[edit]
- “gas” in Diccionari de la llengua catalana, segona edició, Institut d’Estudis Catalans.
- “gas”, in Gran Diccionari de la Llengua Catalana, Grup Enciclopèdia Catalana, 2023
- “gas” in Diccionari normatiu valencià, Acadèmia Valenciana de la Llengua.
- “gas” in Diccionari català-valencià-balear, Antoni Maria Alcover and Francesc de Borja Moll, 1962.
Chinese[edit]
Etymology[edit]
From English gas.
Pronunciation[edit]
- Cantonese (Jyutping): ge1 si2
- Cantonese
- (Standard Cantonese, Guangzhou–Hong Kong)+
- Jyutping: ge1 si2
- Yale: gē sí
- Cantonese Pinyin: ge1 si2
- Guangdong Romanization: gé1 xi2
- Sinological IPA (key): /kɛː⁵⁵ siː³⁵/
- (Standard Cantonese, Guangzhou–Hong Kong)+
Noun[edit]
gas
- (Hong Kong Cantonese) gas (fuel)
Derived terms[edit]
|
|
Dutch[edit]
Pronunciation[edit]
- IPA(key): /ɣɑs/
- Hyphenation: gas
- Rhymes: -ɑs
Etymology 1[edit]
Coined by chemist Jan Baptiste van Helmont in Ortus Medicinae (1648), by way of deliberate similarity to Greek χάος (cháos, “chasm, void, chaos”).
Noun[edit]
gas n (plural gassen, diminutive gasje n)
- gas
- liquefied petroleum gas
- Synonyms: autogas, LPG
Derived terms[edit]
- aardgas
- afvalgas
- autogas
- biogas
- blauwzuurgas
- broeikasgas
- campinggas
- chloorgas
- edelgas
- gasaanleg
- gasaansteker
- gasaanval
- gasaarde
- gasachtig
- gasautomaat
- gasbarbecue
- gasbaten
- gasbedrijf
- gasbel
- gasbescherming
- gasboei
- gasbom
- gasboring
- gasbrander
- gasbron
- gasbuis
- gascel
- gascentrale
- gascilinder
- gascoke
- gasdicht
- gasdruk
- gasexplosie
- gasfabriek
- gasfilter
- gasfitter
- gasfles
- gasfornuis
- gasgeiser
- gasgenerator
- gasgloeilicht
- gasgranaat
- gashaard
- gashendel
- gashouder
- gasijs
- gaskachel
- gaskamer
- gaskast
- gasketel
- gasklok
- gaskous
- gaskraan
- gaskroon
- gaslamp
- gaslantaarn
- gasleiding
- gaslek
- gaslicht
- gaslucht
- gasmasker
- gasmengsel
- gasmeter
- gasmotor
- gasnet
- gasnevel
- gasolie
- gasoline
- gasometer
- gasontploffing
- gasoorlog
- gasoven
- gaspatroon
- gaspedaal
- gaspenning
- gaspijp
- gaspistool
- gaspit
- gasplaneet
- gaspook
- gasprijs
- gasproductie
- gasradiator
- gasreserve
- gasreus
- gasrotonde
- gassig
- gasslang
- gassluis
- gasstel
- gastank
- gastarief
- gasthermometer
- gastoestel
- gasturbine
- gasveer
- gasveld
- gasverbruik
- gasvergiftiging
- gasvering
- gasverlichting
- gasverwarming
- gasvlam
- gasvoorraad
- gasvoorziening
- gasvormig
- gasvorming
- gasvrij
- gaswinning
- gaswolk
- gifgas
- knalgas
- kolengas
- lachgas
- lichtgas
- mijngas
- moerasgas
- mosterdgas
- motorgas
- oorlogsgas
- plankgas
- strijdgas
- traangas
- turfgas
- uitlaatgas
- waterstofgas
- zenuwgas
- zuurstofgas
Descendants[edit]
- Afrikaans: gas
- → Caribbean Javanese: gas
- → English: gas
- → French: gaz
- → Moore: gaase
- → Romanian: gaz
- → Turkish: gaz
- → German: Gas
- → Saramaccan: gási
- → West Frisian: gas
Etymology 2[edit]
From Middle Dutch gasse (“unpaved street”), from Middle High German gazze, from Old High German gazza, from Proto-Germanic *gatwǭ.
Noun[edit]
gas f (plural gassen, diminutive gasje n)
- unpaved street
Etymology 3[edit]
See the etymology of the corresponding lemma form.
Verb[edit]
gas
- first-person singular present indicative of gassen
- imperative of gassen
Galician[edit]
Noun[edit]
gas m (plural gases)
- gas
- Synonym: vapor
Derived terms[edit]
- gas nobre
[edit]
- gasoso
Icelandic[edit]
Pronunciation[edit]
- IPA(key): /kaːs/
- Rhymes: -aːs
Etymology 1[edit]
Borrowed from Dutch gas.
Noun[edit]
gas n (genitive singular gass, nominative plural gös)
- gas (state of matter)
Declension[edit]
Derived terms[edit]
- táragas
Etymology 2[edit]
Borrowed from French gaze.
Noun[edit]
gas n (genitive singular gass, no plural)
- gauze
Declension[edit]
Derived terms[edit]
- gasbleia
Anagrams[edit]
- sag
Indonesian[edit]
Etymology[edit]
From Dutch gas (“gas”), a term coined by chemist Jan Baptist van Helmont. Perhaps inspired by geest (“breath, vapour, spirit”) or by chaos (“chaos”), from Ancient Greek χάος (kháos, “chasm, void”).
Pronunciation[edit]
- IPA(key): [ˈɡas]
- Hyphenation: gas
Noun[edit]
gas (plural gas-gas, first-person possessive gasku, second-person possessive gasmu, third-person possessive gasnya)
- gas,
- (chemistry, physics) Matter in a state intermediate between liquid and plasma that can be contained only if it is fully surrounded by a solid (or in a bubble of liquid) (or held together by gravitational pull); it can condense into a liquid, or can (rarely) become a solid directly.
- A flammable gaseous hydrocarbon or hydrocarbon mixture (typically predominantly methane) used as a fuel, e.g. for cooking, heating, electricity generation or as a fuel in internal combustion engines in vehicles.
Derived terms[edit]
- bergas
- mengegas
Compounds[edit]
- gas air
- gas air mata
- gas alam
- gas basah
- gas batu bara
- gas bersin
- gas buang
- gas bumi
- gas gelap
- gas ikutan
- gas karbit
- gas karbon
- gas kecut
- gas kilang
- gas lamban
- gas lembam
- gas lembap
- gas minyak cair
- gas mulia
- gas pemati lemas
- gas pencerna
- gas pencerna anaerob
- gas penyesak napas
- gas racun
- gas rawa
- gas rumah kaca
- gas saraf
- gas sintesis
- gas tanur
- gas toksik
Verb[edit]
gas
- (colloquial) to hit the gas, to accelerate.
- Synonym: mengegas
Further reading[edit]
- “gas” in Kamus Besar Bahasa Indonesia, Jakarta: Language Development and Fostering Agency — Ministry of Education, Culture, Research, and Technology of the Republic Indonesia, 2016.
Interlingua[edit]
Noun[edit]
gas (plural gases)
- gas
Irish[edit]
Etymology[edit]
(This etymology is missing or incomplete. Please add to it, or discuss it at the Etymology scriptorium.)
Pronunciation[edit]
- (Munster) IPA(key): [ɡɑsˠ]
- (Connacht, Ulster) IPA(key): [ɡasˠ]
Noun[edit]
gas m (genitive singular gais, nominative plural gais or gasa)
- stalk, stem
- sprig, shoot, frond
- (figuratively) stripling; scion
Declension[edit]
Derived terms[edit]
- gasach
- gasán
- gasarnach
- gasghreim
- gasóg
- gasra
Mutation[edit]
| Irish mutation | ||
|---|---|---|
| Radical | Lenition | Eclipsis |
| gas | ghas | ngas |
| Note: Some of these forms may be hypothetical. Not every possible mutated form of every word actually occurs. |
Further reading[edit]
- Ó Dónaill, Niall (1977), “gas”, in Foclóir Gaeilge–Béarla, Dublin: An Gúm, →ISBN
- Entries containing “gas” in English-Irish Dictionary, An Gúm, 1959, by Tomás de Bhaldraithe.
- Entries containing “gas” in New English-Irish Dictionary by Foras na Gaeilge.
Italian[edit]
Pronunciation[edit]
- IPA(key): /ˈɡas/
- Rhymes: -as
- Hyphenation: gàs
Noun[edit]
gas m (uncountable)
- gas (state of matter, petroleum)
- carbon dioxide (in fizzy drinks)
- petrol
- Synonym: benzina
- poison gas
[edit]
- gasare
- gasolina
- gassare
- gassificare
- gassista
- gassometro
- gassoso
Further reading[edit]
- gas in Treccani.it – Vocabolario Treccani on line, Istituto dell’Enciclopedia Italiana
Latin[edit]
Etymology[edit]
Coined by chemist Jan Baptist van Helmont (appearing in his Ortus Medicinae as an invariable noun).
Pronunciation[edit]
- (Classical) IPA(key): /ɡas/, [ɡäs̠]
- (Ecclesiastical) IPA(key): /ɡas/, [ɡäs]
Noun[edit]
gas n (genitive gasis); third declension
- (physics) gas (state of matter)
- Synonyms: gasum, gasium
Declension[edit]
Third-declension noun (neuter, imparisyllabic non-i-stem).
| Case | Singular | Plural |
|---|---|---|
| Nominative | gas | gasa |
| Genitive | gasis | gasum |
| Dative | gasī | gasibus |
| Accusative | gas | gasa |
| Ablative | gase | gasibus |
| Vocative | gas | gasa |
Naga Pidgin[edit]
Etymology[edit]
Inherited from Assamese গাছ (gas).
Noun[edit]
gas
- tree
Norman[edit]
Etymology[edit]
From Old French gars, nominative singular form of garçon.
Noun[edit]
gas m (plural gas)
- (Jersey) chap
Norwegian Bokmål[edit]
Etymology[edit]
From French gaze.
Noun[edit]
gas m (definite singular gasen, indefinite plural gaser, definite plural gasene)
- gauze
See also[edit]
- gass
- gås
References[edit]
- “gas” in The Bokmål Dictionary.
Norwegian Nynorsk[edit]
Etymology[edit]
From French gaze.
Noun[edit]
gas m (definite singular gasen, indefinite plural gasar, definite plural gasane)
- gauze
See also[edit]
- gass
- gås
References[edit]
- “gas” in The Nynorsk Dictionary.
Old Saxon[edit]
Alternative forms[edit]
- gōs
Etymology[edit]
From Proto-West Germanic *gans, from Proto-Germanic *gans, from Proto-Indo-European *ǵʰh₂éns.
Noun[edit]
gās f
- a goose
Declension[edit]
Declension of gās (irregular)
Descendants[edit]
- Low German: Goos
Old Swedish[edit]
Etymology[edit]
From Old Norse gás, from Proto-Germanic *gans.
Noun[edit]
gās f
- goose
Declension[edit]
Declension of gās (consonant stem)
Descendants[edit]
- Swedish: gås
Rohingya[edit]
Etymology[edit]
From Sanskrit.
Noun[edit]
gas
- tree
Romagnol[edit]
Etymology[edit]
From Dutch gas (“gas”), invented by Jan Baptiste van Helmont, from Latin chaos (“chaos”).
Pronunciation[edit]
- IPA(key): /ɡas/
Noun[edit]
gas m (plural ghës)
- gas
Serbo-Croatian[edit]
Pronunciation[edit]
- IPA(key): /ɡâːs/
Noun[edit]
gȃs m (Cyrillic spelling га̑с)
- (chiefly Bosnia, Serbia or colloquial) gas (state of matter)
- Synonym: (Croatian) plȋn
- gas (as fuel for combustion engines)
- (figuratively) acceleration
- dȁti gȃs — “give gas”: accelerate
- gas pedal, accelerator
Declension[edit]
Spanish[edit]
Etymology[edit]
Borrowed from Dutch gas, coined by Belgian chemist Jan Baptist van Helmont. Perhaps inspired by Middle Dutch gheest (Modern Dutch geest (“breath, vapour, spirit”), or from Ancient Greek χάος (kháos, “chasm, void”).
Pronunciation[edit]
- IPA(key): /ˈɡas/ [ˈɡas]
- Rhymes: -as
- Syllabification: gas
Noun[edit]
gas m (plural gases)
- gas (matter between liquid and plasma)
- gas (an element or compound in such a state)
- gas (flammable gas used for combustion)
- (in the plural) gas (waste gases trapped in one’s belly)
Derived terms[edit]
- a medio gas
- a todo gas
- agua con gas
- antigás
- biogas
- cámara de gas
- gas de invernadero
- gas hilarante
- gas inerte
- gas lacrimógeno
- gas mostaza
- gas natural
- gas noble
- gasear
- gaseoso
- gasero
- gasificar
- gasómetro
- perder gas
- tanque de gas
- tener gases
[edit]
- gasolina
Further reading[edit]
- “gas”, in Diccionario de la lengua española, Vigésima tercera edición, Real Academia Española, 2014
Anagrams[edit]
- ags, Ags
Swedish[edit]
Etymology[edit]
From Dutch gas.
Pronunciation[edit]
- IPA(key): /ɡɑːs/
Noun[edit]
gas c
- gas; a state of matter
- gas; a compound or element in such a state
- gas; gaseous fuels
- (plural only: gaser) gas; waste gas
- gas pedal, acceleration (compare gaspedal (“gas pedal”) and gasa (“accelerate, hit the gas”))
-
trampa på gasen
- step on the gas
-
gasen i botten
- pedal to the metal
-
Declension[edit]
| Declension of gas | ||||
|---|---|---|---|---|
| Singular | Plural | |||
| Indefinite | Definite | Indefinite | Definite | |
| Nominative | gas | gasen | gaser | gaserna |
| Genitive | gas | gasens | gasers | gasernas |
Derived terms[edit]
- avgas
- biogas
- fordonsgas
- gasa
- gasbehållare
- gasbrand
- gasbrännare
- gasbuss
- gasformig
- gaskammare
- gasklocka
- gaskromatografi
- gasledning
- gaslykta
- gaslåga
- gasmask
- gaspedal
- gasspis
- gassvetsning
- gasturbin
- gasverk
- ge gas
- ha gaser
- kvävgas
- naturgas
- nervgas
- rötgas
- stadsgas
- sumpgas
- syrgas
- tårgas
- vätgas
Anagrams[edit]
- ags, asg
Tagalog[edit]
Pronunciation[edit]
- IPA(key): /ˈɡas/, [ˈɡas]
Etymology 1[edit]
Either from English gas, itself a clipping of gasoline, or a clipping of gasolina.
Alternative forms[edit]
- gaas
Noun[edit]
gas
- gasoline
- Synonym: gasolina
- kerosene; petroleum; gas
- Synonym: petrolyo
Derived terms[edit]
- gasan
- gasera
- gaserahan
Etymology 2[edit]
Either from Spanish gas or English gas, ultimately from Dutch gas.
Noun[edit]
gas
- gaseous substance; vapor; fume
- Synonyms: singaw, asngaw
Welsh[edit]
Pronunciation[edit]
- IPA(key): /ɡaːs/
Verb[edit]
gas
- Soft mutation of cas.
Mutation[edit]
| Welsh mutation | |||
|---|---|---|---|
| radical | soft | nasal | aspirate |
| cas | gas | nghas | chas |
| Note: Some of these forms may be hypothetical. Not every possible mutated form of every word actually occurs. |
West Frisian[edit]
Etymology[edit]
Borrowed from Dutch gas.
Pronunciation[edit]
- IPA(key): /ɡɔs/
Noun[edit]
gas n (plural gassen)
- gas
Further reading[edit]
- “gas”, in Wurdboek fan de Fryske taal (in Dutch), 2011
- Top Definitions
- Quiz
- Related Content
- Examples
- British
- Scientific
- Cultural
- Idioms And Phrases
This shows grade level based on the word’s complexity.
This shows grade level based on the word’s complexity.
noun, plural gas·es or gas·ses.
Physics. a substance possessing perfect molecular mobility and the property of indefinite expansion, as opposed to a solid or liquid.
any such fluid or mixture of fluids.
any such fluid used as an anesthetic, as nitrous oxide: Did the dentist give you gas for your extraction?
any such combustible fluid used as fuel: Light the gas in the oven.
Automotive.
- gasoline.
- Also called gas pedal . the foot-operated accelerator of an automotive vehicle: Take your foot off the gas.
Coal Mining. an explosive mixture of firedamp with air.
an aeriform fluid or a mistlike assemblage of fine particles suspended in air, used in warfare to asphyxiate, poison, or stupefy an enemy.
Slang.
- empty talk.
- a person or thing that is very entertaining, pleasing, or successful: The party was an absolute gas, and we loved it.
- a person or thing that affects one strongly.
verb (used with object), gassed, gas·sing.
to supply with gas.
to overcome, poison, or asphyxiate with gas or fumes.
to singe (yarns or fabrics) with a gas flame to remove superfluous fibers.
to treat or impregnate with gas.
Slang.
- to talk nonsense or falsehood to.
- to amuse or affect strongly: Her weird clothes really gas me.
verb (used without object), gassed, gas·sing.
to give off gas, as a storage battery being charged.
Slang.
- to indulge in idle, empty talk.
- to become drunk (often followed by up).
Verb Phrases
gas up, to fill the gasoline tank of an automobile, truck, or other vehicle.
QUIZ
CAN YOU ANSWER THESE COMMON GRAMMAR DEBATES?
There are grammar debates that never die; and the ones highlighted in the questions in this quiz are sure to rile everyone up once again. Do you know how to answer the questions that cause some of the greatest grammar debates?
Which sentence is correct?
Idioms about gas
step on the gas, Informal. to increase the speed of one’s movement or activity; hurry: We’d better step on the gas or we’ll be late for the concert.
Origin of gas
First recorded in 1650–60; coined by J. B. van Helmont (1577–1644), Flemish chemist; suggested by Greek cháos “atmosphere”
OTHER WORDS FROM gas
gasless, adjectivenon·gas, noun, plural non·gas·es.
Words nearby gas
garter stitch, garth, garvey, Garvey, Marcus, Gary, gas, gas bacillus, gasbag, gas black, gas burner, gas chamber
Dictionary.com Unabridged
Based on the Random House Unabridged Dictionary, © Random House, Inc. 2023
Words related to gas
How to use gas in a sentence
-
In his testimony, DeMarco said he found spent tear gas canisters on the street after protesters were cleared.
-
As greenhouse gas emissions warm the planet, the ocean is absorbing vast amounts of that heat.
-
The trouble is, it can be genuinely hard to figure out how to direct your money wisely if you want to reduce greenhouse gas emissions.
-
On the surface, it’s a triangular tussle involving Turkey, Greece and Cyprus over potentially giant gas reserves deep under the Mediterranean Sea.
-
The gas is produced by microbes here on Earth, and after most known nonbiological processes were ruled out, the discovery has renewed hopes that there’s life on Venus.
-
Security officials told Agence France-Presse that the gas station manager said he had recognized the two men.
-
Unconfirmed reports in the French media claimed that the brothers were spotted at a gas station in northern France on Thursday.
-
Total oil production figures include crude oil, natural gas liquids, and other liquid energy products.
-
On top of oil, the United States produces significantly more natural gas than Saudi Arabia.
-
Her slight miscalculation of how to fix the situation leads to her driving around the gas pump.
-
For comparison, the gas may be passed through a test-tube containing an equal amount of distilled water.
-
It is allowed to cool, and hydrogen sulphid gas is passed through it for about five minutes.
-
You could not see the water till you got close up, and at a distance only the rows of gas jets were apparent.
-
Gas, it is clear, could not be carried into a hostile country or into remote and nearly inaccessible districts.
-
In its pure state it is a transparent and colourless gas, having a peculiar pungent smell, and highly soluble in water.
British Dictionary definitions for gas
noun plural gases or gasses
a substance in a physical state in which it does not resist change of shape and will expand indefinitely to fill any container. If very high pressure is applied a gas may become liquid or solid, otherwise its density tends towards that of the condensed phaseCompare liquid (def. 1), solid (def. 1)
any substance that is gaseous at room temperature and atmospheric pressure
any gaseous substance that is above its critical temperature and therefore not liquefiable by pressure aloneCompare vapour (def. 2)
- a fossil fuel in the form of a gas, used as a source of domestic and industrial heatSee also coal gas, natural gas
- (as modifier)a gas cooker; gas fire
a gaseous anaesthetic, such as nitrous oxide
mining firedamp or the explosive mixture of firedamp and air
the usual US, Canadian, and New Zealand word for petrol See also gasoline
step on the gas informal
- to increase the speed of a motor vehicle; accelerate
- to hurry
a toxic or suffocating substance in suspension in air used against an enemy
informal idle talk or boasting
slang a delightful or successful person or thinghis latest record is a gas
US an informal name for flatus
verb gases, gasses, gassing or gassed
(tr) to provide or fill with gas
(tr) to subject to gas fumes, esp so as to asphyxiate or render unconscious
(intr) to give off gas, as in the charging of a battery
(tr) (in textiles) to singe (fabric) with a flame from a gas burner to remove unwanted fibres
(intr foll by to) informal to talk in an idle or boastful way (to a person)
(tr) slang, mainly US and Canadian to thrill or delight
Derived forms of gas
gasless, adjective
Word Origin for gas
C17 (coined by J. B. van Helmont (1577–1644), Flemish chemist): modification of Greek khaos atmosphere
Collins English Dictionary — Complete & Unabridged 2012 Digital Edition
© William Collins Sons & Co. Ltd. 1979, 1986 © HarperCollins
Publishers 1998, 2000, 2003, 2005, 2006, 2007, 2009, 2012
Scientific definitions for gas
One of four main states of matter, composed of molecules in constant random motion. Unlike a solid, a gas has no fixed shape and will take on the shape of the space available. Unlike a liquid, the intermolecular forces are very small; it has no fixed volume and will expand to fill the space available.
Other words from gas
gaseous adjective (găs′ē-əs, găsh′əs)
The American Heritage® Science Dictionary
Copyright © 2011. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.
Cultural definitions for gas
The New Dictionary of Cultural Literacy, Third Edition
Copyright © 2005 by Houghton Mifflin Harcourt Publishing Company. Published by Houghton Mifflin Harcourt Publishing Company. All rights reserved.
Other Idioms and Phrases with gas
In addition to the idiom beginning with gas
- gas up
also see:
- cook with gas
- run out of steam (gas)
The American Heritage® Idioms Dictionary
Copyright © 2002, 2001, 1995 by Houghton Mifflin Harcourt Publishing Company. Published by Houghton Mifflin Harcourt Publishing Company.