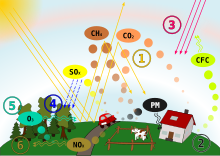
Acid clouds can grow on SO2 emissions from refineries, as seen here in Curaçao.
| External audio |
|---|
Acid rain is rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). Most water, including drinking water, has a neutral pH that exists between 6.5 and 8.5, but acid rain has a pH level lower than this and ranges from 4–5 on average.[1][2] The more acidic the acid rain is, the lower its pH is.[2] Acid rain can have harmful effects on plants, aquatic animals, and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids.
Acid rain has been shown to have adverse impacts on forests, freshwaters, soils, microbes, insects and aquatic life-forms.[3] In ecosystems, persistent acid rain reduces tree bark durability, leaving flora more susceptible to environmental stressors such as drought, heat/cold and pest infestation. Acid rain is also capable of detrimenting soil composition by stripping it of nutrients such as calcium and magnesium which play a role in plant growth and maintaining healthy soil. In terms of human infrastructure, acid rain also causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues as well as having impacts on human health.[4][5][6][7]
Some governments, including those in Europe and North America, have made efforts since the 1970s to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere through air pollution regulations. These efforts have had positive results due to the widespread research on acid rain starting in the 1960s and the publicized information on its harmful effects.[8][9] The main source of sulfur and nitrogen compounds that result in acid rain are anthropogenic, but nitrogen oxides can also be produced naturally by lightning strikes and sulphur dioxide is produced by volcanic eruptions.[10]
Definition
«Acid rain» is a popular term referring to the deposition of a mixture from wet (rain, snow, sleet, fog, cloudwater, and dew) and dry (acidifying particles and gases) acidic components. Distilled water, once carbon dioxide is removed, has a neutral pH of 7.[11] Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are alkaline. «Clean» or unpolluted rain has an acidic pH, but usually no lower than 5.7, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid according to the following reaction:
- H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
Carbonic acid then can ionize in water forming low concentrations of carbonate and hydronium ions:
- H2O (l) + H2CO3 (aq) ⇌ HCO−3 (aq) + H3O+ (aq)
Unpolluted rain can also contain other chemicals which affect its pH (acidity level). A common example is nitric acid produced by electric discharge in the atmosphere such as lightning.[12] Acid deposition as an environmental issue (discussed later in the article) would include additional acids other than H2CO3.
Occasional pH readings in rain and fog water of well below 2.4 have been reported in industrialized areas.[13]
History
Acid rain was first systematically studied in Europe, in the 1960s, and in the United States and Canada, the following decade.
In Europe
The corrosive effect of polluted, acidic city air on limestone and marble was noted in the 17th century by John Evelyn, who remarked upon the poor condition of the Arundel marbles.[14]
Since the Industrial Revolution, emissions of sulfur dioxide and nitrogen oxides into the atmosphere have increased.[13][15] In 1852, Robert Angus Smith was the first to show the relationship between acid rain and atmospheric pollution in Manchester, England.[16] Smith coined the term «acid rain» in 1872.[17]
In the late 1960s, scientists began widely observing and studying the phenomenon.[18] At first, the main focus in this research lay on local effects of acid rain. Waldemar Christofer Brøgger was the first to acknowledge long-distance transportation of pollutants crossing borders from the United Kingdom to Norway – a problem systematically studied by Brynjulf Ottar in the 1970s.[19] Ottar’s work was strongly influenced[20] by Swedish soil scientist Svante Odén, who had drawn widespread attention to Europe’s acid rain problem in popular newspapers and wrote a landmark paper on the subject in 1968.[21][22][23]
In the United States
| External audio |
|---|
 |
Since 1998, Harvard University wraps some of the bronze and marble statues on its campus, such as this «Chinese stele», with waterproof covers every winter, in order to protect them from corrosion caused by acid rain and acid snow[24]
The earliest report about acid rain in the United States came from chemical evidence gathered from Hubbard Brook Valley; public awareness of acid rain in the US increased in the 1970s after The New York Times reported on these findings.[25][26]
In 1972, a group of scientists including Gene Likens discovered the rain that was deposited at White Mountains of New Hampshire was acidic. The pH of the sample was measured to be 4.03 at Hubbard Brook.[27] The Hubbard Brook Ecosystem Study followed up with a series of research studies that analyzed the environmental effects of acid rain. Acid rain that mixed with stream water at Hubbard Brook was neutralized by the alumina from soils.[28] The result of this research indicated that the chemical reaction between acid rain and aluminum leads to an increasing rate of soil weathering. Experimental research was done to examine the effects of increased acidity in streams on ecological species. In 1980, a group of scientists modified the acidity of Norris Brook, New Hampshire, and observed the change in species’ behaviors. There was a decrease in species diversity, an increase in community dominants, and a decrease in the food web complexity.[29]
In 1980, the US Congress passed an Acid Deposition Act.[30] This Act established an 18-year assessment and research program under the direction of the National Acidic Precipitation Assessment Program (NAPAP). NAPAP enlarged a network of monitoring sites to determine how acidic the precipitation actually was, seeking to determine long-term trends, and established a network for dry deposition. Using a statistically based sampling design, NAPAP quantified the effects of acid rain on a regional basis by targeting research and surveys to identify and quantify the effects of acid precipitation on freshwater and terrestrial ecosystems. NAPAP also assessed the effects of acid rain on historical buildings, monuments, and building materials. It also funded extensive studies on atmospheric processes and potential control programs.
From the start, policy advocates from all sides attempted to influence NAPAP activities to support their particular policy advocacy efforts, or to disparage those of their opponents.[30] For the US Government’s scientific enterprise, a significant impact of NAPAP were lessons learned in the assessment process and in environmental research management to a relatively large group of scientists, program managers, and the public.[31]
In 1981, the National Academy of Sciences was looking into research about the controversial issues regarding acid rain.[32] President Ronald Reagan dismissed the issues of acid rain[33] until his personal visit to Canada and confirmed that the Canadian border suffered from the drifting pollution from smokestacks originating in the US Midwest. Reagan honored the agreement to Canadian Prime Minister Pierre Trudeau’s enforcement of anti-pollution regulation.[34] In 1982, Reagan commissioned William Nierenberg to serve on the National Science Board.[35] Nierenberg selected scientists including Gene Likens to serve on a panel to draft a report on acid rain. In 1983, the panel of scientists came up with a draft report, which concluded that acid rain is a real problem and solutions should be sought.[36] White House Office of Science and Technology Policy reviewed the draft report and sent Fred Singer’s suggestions of the report, which cast doubt on the cause of acid rain.[37] The panelists revealed rejections against Singer’s positions and submitted the report to Nierenberg in April. In May 1983, the House of Representatives voted against legislation that aimed to control sulfur emissions. There was a debate about whether Nierenberg delayed to release the report. Nierenberg himself denied the saying about his suppression of the report and stated that the report was withheld after the House’s vote because it was not ready to be published.[38]
In 1991, the US National Acid Precipitation Assessment Program (NAPAP) provided its first assessment of acid rain in the United States.[39] It reported that 5% of New England Lakes were acidic, with sulfates being the most common problem. They noted that 2% of the lakes could no longer support Brook Trout, and 6% of the lakes were unsuitable for the survival of many species of minnow. Subsequent Reports to Congress have documented chemical changes in soil and freshwater ecosystems, nitrogen saturation, decreases in amounts of nutrients in soil, episodic acidification, regional haze, and damage to historical monuments.
Meanwhile, in 1990, the US Congress passed a series of amendments to the Clean Air Act.[40] Title IV of these amendments established a cap and trade system designed to control emissions of sulfur dioxide and nitrogen oxides.[41] Title IV called for a total reduction of about 10 million tons of SO2 emissions from power plants, close to a 50% reduction.[41] It was implemented in two phases. Phase I began in 1995, and limited sulfur dioxide emissions from 110 of the largest power plants to a combined total of 8.7 million tons of sulfur dioxide. One power plant in New England (Merrimack) was in Phase I. Four other plants (Newington, Mount Tom, Brayton Point, and Salem Harbor) were added under other provisions of the program. Phase II began in 2000, and affects most of the power plants in the country.
During the 1990s, research continued. On March 10, 2005, the EPA issued the Clean Air Interstate Rule (CAIR). This rule provides states with a solution to the problem of power plant pollution that drifts from one state to another. CAIR will permanently cap emissions of SO2 and NOx in the eastern United States. When fully implemented[when?], CAIR will reduce SO2 emissions in 28 eastern states and the District of Columbia by over 70% and NOx emissions by over 60% from 2003 levels.[42]
Overall, the program’s cap and trade program has been successful in achieving its goals. Since the 1990s, SO2 emissions have dropped 40%, and according to the Pacific Research Institute, acid rain levels have dropped 65% since 1976.[43][44] Conventional regulation was used in the European Union, which saw a decrease of over 70% in SO2 emissions during the same time period.[45]
In 2007, total SO2 emissions were 8.9 million tons, achieving the program’s long-term goal ahead of the 2010 statutory deadline.[46]
In 2007 the EPA estimated that by 2010, the overall costs of complying with the program for businesses and consumers would be $1 billion to $2 billion a year, only one fourth of what was originally predicted.[43] Forbes says: «In 2010, by which time the cap and trade system had been augmented by the George W. Bush administration’s Clean Air Interstate Rule, SO2 emissions had fallen to 5.1 million tons.»[47]
The term citizen science can be traced back as far as January 1989 to a campaign by the Audubon Society to measure acid rain. Scientist Muki Haklay cites in a policy report for the Wilson Center entitled ‘Citizen Science and Policy: A European Perspective’ a first use of the term ‘citizen science’ by R. Kerson in the magazine MIT Technology Review from January 1989.[48][49] Quoting from the Wilson Center report: «The new form of engagement in science received the name «citizen science». The first recorded example of the use of the term is from 1989, describing how 225 volunteers across the US collected rain samples to assist the Audubon Society in an acid-rain awareness raising campaign. The volunteers collected samples, checked for acidity, and reported back to the organization. The information was then used to demonstrate the full extent of the phenomenon.»[48][49]
In Canada
Canadian Harold Harvey was among the first to research a «dead» lake. In 1971, he and R.J. Beamish published a report, «Acidification of the La Cloche Mountain Lakes», documenting the gradual deterioration of fish stocks in 60 lakes in Killarney Park in Ontario, which they had been studying systematically since 1966.[50]
In the 1970s and 80s, acid rain was a major topic of research at the Experimental Lakes Area (ELA) in Northwestern Ontario, Canada.[51] Researchers added sulfuric acid to whole lakes in controlled ecosystem experiments to simulate the effects of acid rain. Because its remote conditions allowed for whole-ecosystem experiments, research at the ELA showed that the effect of acid rain on fish populations started at concentrations much lower than those observed in laboratory experiments.[52] In the context of a food web, fish populations crashed earlier than when acid rain had direct toxic effects to the fish because the acidity led to crashes in prey populations (e.g. mysids).[52] As experimental acid inputs were reduced, fish populations and lake ecosystems recovered at least partially, although invertebrate populations have still not completely returned to the baseline conditions.[53] This research showed both that acidification was linked to declining fish populations and that the effects could be reversed if sulfuric acid emissions decreased, and influenced policy in Canada and the United States.[51]
In 1985, seven Canadian provinces (all except British Columbia, Alberta, and Saskatchewan) and the federal government signed the Eastern Canada Acid Rain Program.[54] The provinces agreed to limit their combined sulfur dioxide emissions to 2.3 million tonnes by 1994. The Canada-US Air Quality Agreement was signed in 1991.[54] In 1998, all federal, provincial, and territorial Ministers of Energy and Environment signed The Canada-Wide Acid Rain Strategy for Post-2000, which was designed to protect lakes that are more sensitive than those protected by earlier policies.[54]
Emissions of chemicals leading to acidification
The most important gas which leads to acidification is sulfur dioxide. Emissions of nitrogen oxides which are oxidized to form nitric acid are of increasing importance due to stricter controls on emissions of sulfur compounds. 70 Tg(S) per year in the form of SO2 comes from fossil fuel combustion and industry, 2.8 Tg(S) from wildfires, and 7–8 Tg(S) per year from volcanoes.[55]
Natural phenomena
| Food Types | Acidifying Emissions (g SO2eq per 100g protein) |
|---|---|
| Beef |
343.6 |
| Cheese |
165.5 |
| Pork |
142.7 |
| Lamb and Mutton |
139.0 |
| Farmed Crustaceans |
133.1 |
| Poultry |
102.4 |
| Farmed Fish |
65.9 |
| Eggs |
53.7 |
| Groundnuts |
22.6 |
| Peas |
8.5 |
| Tofu |
6.7 |
The principal natural phenomena that contribute acid-producing gases to the atmosphere are emissions from volcanoes.[57] Thus, for example, fumaroles from the Laguna Caliente crater of Poás Volcano create extremely high amounts of acid rain and fog, with acidity as high as a pH of 2, clearing an area of any vegetation and frequently causing irritation to the eyes and lungs of inhabitants in nearby settlements. Acid-producing gasses are also created by biological processes that occur on the land, in wetlands, and in the oceans. The major biological source of sulfur compounds is dimethyl sulfide.
Nitric acid in rainwater is an important source of fixed nitrogen for plant life, and is also produced by electrical activity in the atmosphere such as lightning.[58]
Acidic deposits have been detected in glacial ice thousands of years old in remote parts of the globe.[59]
Human activity
The principal cause of acid rain is sulfur and nitrogen compounds from human sources, such as electricity generation, animal agriculture, factories, and motor vehicles. Industrial acid rain is a substantial problem in China and Russia[60][61] and areas downwind from them. These areas all burn sulfur-containing coal to generate heat and electricity.[62]
The problem of acid rain has not only increased with population and industrial growth, but has become more widespread. The use of tall smokestacks to reduce local pollution has contributed to the spread of acid rain by releasing gases into regional atmospheric circulation; dispersal from these taller stacks causes pollutants to be carried farther, causing widespread ecological damage.[59][63] Often deposition occurs a considerable distance downwind of the emissions, with mountainous regions tending to receive the greatest deposition (because of their higher rainfall). An example of this effect is the low pH of rain which falls in Scandinavia.
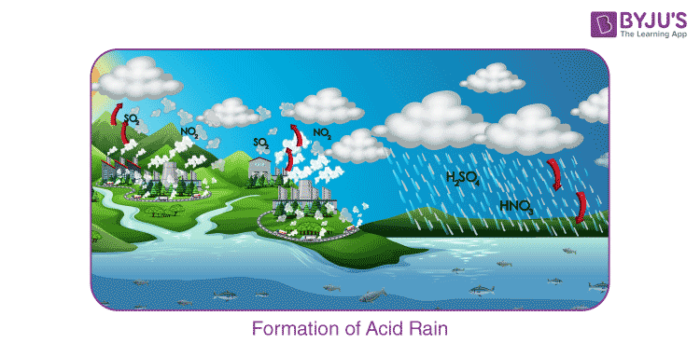
Chemical processes
Combustion of fuels produces sulfur dioxide and nitric oxides. They are converted into sulfuric acid and nitric acid.[64]
Gas phase chemistry
In the gas phase sulfur dioxide is oxidized by reaction with the hydroxyl radical via an intermolecular reaction:[16]
- SO2 + OH· → HOSO2·
which is followed by:
- HOSO2· + O2 → HO2· + SO3
In the presence of water, sulfur trioxide (SO3) is converted rapidly to sulfuric acid:
- SO3 (g) + H2O (l) → H2SO4 (aq)
Nitrogen dioxide reacts with OH to form nitric acid:
This shows the process of the air pollution being released into the atmosphere and the areas that will be affected.
- NO2 + OH· → HNO3
Chemistry in cloud droplets
When clouds are present, the loss rate of SO2 is faster than can be explained by gas phase chemistry alone. This is due to reactions in the liquid water droplets.
- Hydrolysis
Sulfur dioxide dissolves in water and then, like carbon dioxide, hydrolyses in a series of equilibrium reactions:
- SO2 (g) + H2O ⇌ SO2·H2O
- SO2·H2O ⇌ H+ + HSO3−
- HSO3− ⇌ H+ + SO32−
- Oxidation
There are a large number of aqueous reactions that oxidize sulfur from S(IV) to S(VI), leading to the formation of sulfuric acid. The most important oxidation reactions are with ozone, hydrogen peroxide and oxygen (reactions with oxygen are catalyzed by iron and manganese in the cloud droplets).[16]
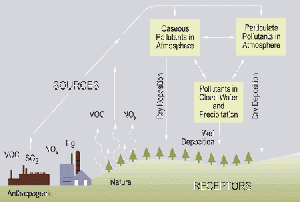
Acid deposition
Wet deposition
Wet deposition of acids occurs when any form of precipitation (rain, snow, and so on) removes acids from the atmosphere and delivers it to the Earth’s surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosols are both of importance for wet deposition.[65]
Dry deposition
Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20 to 60% of total acid deposition.[66] This occurs when particles and gases stick to the ground, plants or other surfaces.[65]
Adverse effects
Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms as well as causing damage to buildings and having impacts on human health.
Surface waters and aquatic animals
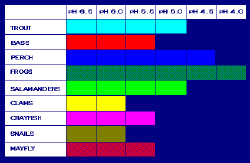
Not all fish, shellfish, or the insects that they eat can tolerate the same amount of acid; for example, frogs can tolerate water that is more acidic (i.e., has a lower pH) than trout.
Both the lower pH and higher aluminium concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pH lower than 5 most fish eggs will not hatch and lower pH can kill adult fish. As lakes and rivers become more acidic biodiversity is reduced. Acid rain has eliminated insect life and some fish species, including the brook trout in some lakes, streams, and creeks in geographically sensitive areas, such as the Adirondack Mountains of the United States.[67] However, the extent to which acid rain contributes directly or indirectly via runoff from the catchment to lake and river acidity (i.e., depending on characteristics of the surrounding watershed) is variable. The United States Environmental Protection Agency’s (EPA) website states: «Of the lakes and streams surveyed, acid rain caused acidity in 75% of the acidic lakes and about 50% of the acidic streams».[67] Lakes hosted by silicate basement rocks are more acidic than lakes within limestone or other basement rocks with a carbonate composition (i.e. marble) due to buffering effects by carbonate minerals, even with the same amount of acid rain.[68][citation needed]
Soils
Soil biology and chemistry can be seriously damaged by acid rain. Some microbes are unable to tolerate changes to low pH and are killed.[69] The enzymes of these microbes are denatured (changed in shape so they no longer function) by the acid. The hydronium ions of acid rain also mobilize toxins, such as aluminium, and leach away essential nutrients and minerals such as magnesium.[5]
- 2 H+ (aq) + Mg2+ (clay) ⇌ 2 H+ (clay) + Mg2+ (aq)
Soil chemistry can be dramatically changed when base cations, such as calcium and magnesium, are leached by acid rain, thereby affecting sensitive species, such as sugar maple (Acer saccharum).[70]
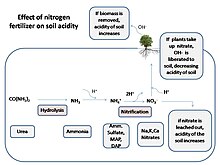
Soil acidification
Diagram of nutrient leaching in soil with high levels of Soil acidity.
Impacts of acidic water and Soil acidification on plants could be minor or in most cases major. Most minor cases which do not result in fatality of plant life can be attributed to the plants being less susceptible to acidic conditions and/or the acid rain being less potent. However, even in minor cases, the plant will eventually die due to the acidic water lowering the plant’s natural pH.[71] Acidic water enters the plant and causes important plant minerals to dissolve and get carried away; which ultimately causes the plant to die of lack of minerals for nutrition. In major cases, which are more extreme, the same process of damage occurs as in minor cases, which is removal of essential minerals, but at a much quicker rate.[6] Likewise, acid rain that falls on soil and on plant leaves causes drying of the waxy leaf cuticle, which ultimately causes rapid water loss from the plant to the outside atmosphere and eventually results in death of the plant.[72] To see if a plant is being affected by soil acidification, one can closely observe the plant leaves. If the leaves are green and look healthy, the soil pH is normal and acceptable for plant life. But if the plant leaves have yellowing between the veins on their leaves, that means the plant is suffering from acidification and is unhealthy.[73] Moreover, a plant suffering from soil acidification cannot photosynthesize; the acid-water-induced process of drying out of the plant can destroy chloroplast organelles.[74] Without being able to photosynthesize, a plant cannot create nutrients for its own survival or oxygen for the survival of aerobic organisms, which affects most species on Earth and ultimately ends the purpose of the plant’s existence.[75]
Forests and other vegetation
Acid rain can have severe effects on vegetation. A forest in the Black Triangle in Europe.
Adverse effects may be indirectly related to acid rain, like the acid’s effects on soil (see above) or high concentration of gaseous precursors to acid rain. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.[76]
Other plants can also be damaged by acid rain, but the effect on food crops is minimized by the application of lime and fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. When calcium is leached from the needles of red spruce, these trees become less cold tolerant and exhibit winter injury and even death.[77][78]
Ocean acidification
|
This section needs expansion. You can help by adding to it. (July 2013) |
Acid rain has a much less harmful effect on oceans on a global scale, but it creates an amplified impact in the shallower waters of coastal waters.[79] Acid rain can cause the ocean’s pH to fall, known as ocean acidification, making it more difficult for different coastal species to create their exoskeletons that they need to survive. These coastal species link together as part of the ocean’s food chain, and without them being a source for other marine life to feed off of, more marine life will die.[80] Coral’s limestone skeleton is particularly sensitive to pH decreases, because the calcium carbonate, a core component of the limestone skeleton, dissolves in acidic (low pH) solutions.
In addition to acidification, excess nitrogen inputs from the atmosphere promote increased growth of phytoplankton and other marine plants, which, in turn, may cause more frequent harmful algal blooms and eutrophication (the creation of oxygen-depleted «dead zones») in some parts of the ocean.[79]
Human health effects
Acid rain does not directly affect human health. The acid in the rainwater is too dilute to have direct adverse effects. The particulates responsible for acid rain (sulfur dioxide and nitrogen oxides) do have an adverse effect. Increased amounts of fine particulate matter in the air contribute to heart and lung problems, including asthma and bronchitis.[7]
Other adverse effects
Effect of acid rain on statues
Acid rain can damage buildings, historic monuments, and statues, especially those made of rocks, such as limestone and marble, that contain large amounts of calcium carbonate. Acids in the rain react with the calcium compounds in the stones to create gypsum, which then flakes off.
- CaCO3 (s) + H2SO4 (aq) ⇌ CaSO4 (s) + CO2 (g) + H2O (l)
The effects of this are commonly seen on old gravestones, where acid rain can cause the inscriptions to become completely illegible. Acid rain also increases the corrosion rate of metals, in particular iron, steel, copper and bronze.[81][82]
Affected areas
Places significantly impacted by acid rain around the globe include most of eastern Europe from Poland northward into Scandinavia,[83] the eastern third of the United States,[84] and southeastern Canada. Other affected areas include the southeastern coast of China and Taiwan.[85]
Prevention methods
Technical solutions
Many coal-firing power stations use flue-gas desulfurization (FGD) to remove sulfur-containing gases from their stack gases. For a typical coal-fired power station, FGD will remove 95% or more of the SO2 in the flue gases. An example of FGD is the wet scrubber which is commonly used. A wet scrubber is basically a reaction tower equipped with a fan that extracts hot smoke stack gases from a power plant into the tower. Lime or limestone in slurry form is also injected into the tower to mix with the stack gases and combine with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-neutral calcium sulfate that is physically removed from the scrubber. That is, the scrubber turns sulfur pollution into industrial sulfates.
In some areas the sulfates are sold to chemical companies as gypsum when the purity of calcium sulfate is high. In others, they are placed in landfill. The effects of acid rain can last for generations, as the effects of pH level change can stimulate the continued leaching of undesirable chemicals into otherwise pristine water sources, killing off vulnerable insect and fish species and blocking efforts to restore native life.
Fluidized bed combustion also reduces the amount of sulfur emitted by power production.
Vehicle emissions control reduces emissions of nitrogen oxides from motor vehicles.
International treaties
Governmental action to combat the effects of acid rain
International treaties on the long-range transport of atmospheric pollutants have been agreed upon by western countries for some time now. Beginning in 1979, European countries convened in order to ratify general principles discussed during the UNECE Convention. The purpose was to combat Long-Range Transboundary Air Pollution.[86] The 1985 Helsinki Protocol on the Reduction of Sulphur Emissions under the Convention on Long-Range Transboundary Air Pollution furthered the results of the convention. Results of the treaty have already come to fruition, as evidenced by an approximate 40 percent drop in particulate matter in North America.[87] The effectiveness of the Convention in combatting acid rain has inspired further acts of international commitment to prevent the proliferation of particulate matter. Canada and the US signed the Air Quality Agreement in 1991. Most European countries and Canada signed the treaties. Activity of the Long-Range Transboundary Air Pollution Convention remained dormant after 1999, when 27 countries convened to further reduce the effects of acid rain.[88] In 2000, foreign cooperation to prevent acid rain was sparked in Asia for the first time. Ten diplomats from countries ranging throughout the continent convened to discuss ways to prevent acid rain.[89] Following these discussions, the Acid Deposition Monitoring Network in East Asia (EANET) was established in 2001 as an intergovernmental initiative to provide science-based inputs for decision makers and promote international cooperation on acid deposition in East Asia.[90] In 2023, the EANET member countries include Cambodia, China, Indonesia, Japan, Lao PDR, Malaysia, Mongolia, Myanmar, the Philippines, Republic of Korea, Russia, Thailand and Vietnam.[91]
Emissions trading
In this regulatory scheme, every current polluting facility is given or may purchase on an open market an emissions allowance for each unit of a designated pollutant it emits. Operators can then install pollution control equipment, and sell portions of their emissions allowances they no longer need for their own operations, thereby recovering some of the capital cost of their investment in such equipment. The intention is to give operators economic incentives to install pollution controls.
The first emissions trading market was established in the United States by enactment of the Clean Air Act Amendments of 1990.[92] The overall goal of the Acid Rain Program established by the Act[93] is to achieve significant environmental and public health benefits through reductions in emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx), the primary causes of acid rain. To achieve this goal at the lowest cost to society, the program employs both regulatory and market based approaches for controlling air pollution.
See also
- Alkaline precipitation
- Citizen science — one of two ‘first uses’ of the term was in an acid rain campaign 1989.
- Gene Likens
- List of environmental issues
- Lists of environmental topics
- Ocean acidification
- Rain dust (an alkaline rain)
- Soil retrogression and degradation
References
- ^ US EPA, OW (September 3, 2015). «Drinking Water Regulations and Contaminants». www.epa.gov. Retrieved October 19, 2021.
- ^ a b US EPA, OAR (February 9, 2016). «What is Acid Rain?». www.epa.gov. Retrieved October 19, 2021.
- ^ US EPA, OAR (March 16, 2016). «Effects of Acid Rain». www.epa.gov. Retrieved March 29, 2022.
- ^ Magaino, S. (January 1, 1997). «Corrosion rate of copper rotating-disk-electrode in simulated acid rain». Electrochimica Acta. 42 (3): 377–382. doi:10.1016/S0013-4686(96)00225-3. ISSN 0013-4686. Archived from the original on June 9, 2020. Retrieved April 22, 2020.
- ^ a b US EPA: Effects of Acid Rain – Forests Archived July 26, 2008, at the Wayback Machine
- ^ a b Markewitz, Daniel; Richter, Daniel D.; Allen, H. Lee; Urrego, J. Byron (1998). «Three Decades of Observed Soil Acidification in the Calhoun Experimental Forest: Has Acid Rain Made a Difference?». Soil Science Society of America Journal. 62 (5): 1428–1439. Bibcode:1998SSASJ..62.1428M. doi:10.2136/sssaj1998.03615995006200050040x. ISSN 1435-0661.
- ^ a b Effects of Acid Rain – Human Health Archived January 18, 2008, at the Wayback Machine. Epa.gov (June 2, 2006). Retrieved on 2013-02-09.
- ^ P. Rafferty, John. «What Happened to Acid Rain?». Encyclopædia Britannica. Retrieved July 21, 2022.
- ^ Kjellstrom, Tord; Lodh, Madhumita; McMichael, Tony; Ranmuthugala, Geetha; Shrestha, Rupendra; Kingsland, Sally (2006), Jamison, Dean T.; Breman, Joel G.; Measham, Anthony R.; Alleyne, George (eds.), «Air and Water Pollution: Burden and Strategies for Control», Disease Control Priorities in Developing Countries (2nd ed.), World Bank, ISBN 978-0-8213-6179-5, PMID 21250344, archived from the original on August 7, 2020, retrieved April 22, 2020
- ^ Sisterson, D. L.; Liaw, Y. P. (January 1, 1990). «An evaluation of lightning and corona discharge on thunderstorm air and precipitation chemistry». Journal of Atmospheric Chemistry. 10 (1): 83–96. Bibcode:1990JAtC…10…83S. doi:10.1007/BF01980039. ISSN 1573-0662. S2CID 97714446.
- ^ US EPA, OAR (February 9, 2016). «What is Acid Rain?». www.epa.gov. Retrieved September 13, 2021.
- ^ Likens, Gene E.; Keene, William C.; Miller, John M.; Galloway, James N. (1987). «Chemistry of precipitation from a remote, terrestrial site in Australia». Journal of Geophysical Research. 92 (D11): 13299. Bibcode:1987JGR….9213299L. doi:10.1029/JD092iD11p13299. Archived from the original on February 21, 2021. Retrieved August 25, 2020.
- ^ a b Glossary, United States: NASA Earth Observatory, acid rain, archived from the original on December 13, 2011, retrieved February 15, 2013
- ^ E. S. de Beer, ed. The Diary of John Evelyn, III, 1955 (September 19, 1667) p. 495.
- ^ Weathers, K. C. and Likens, G. E. (2006). «Acid rain», pp. 1549–1561 in: W. N. Rom and S. Markowitz (eds.). Environmental and Occupational Medicine. Lippincott-Raven Publ., Philadelphia. Fourth Edition, ISBN 0-7817-6299-5.
- ^ a b c Seinfeld, John H.; Pandis, Spyros N (1998). Atmospheric Chemistry and Physics — From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 978-0-471-17816-3
- ^ Acid Rain in New England, A Brief History Archived September 25, 2010, at the Wayback Machine. Epa.gov. Retrieved on February 9, 2013.
- ^ Likens, G. E.; Bormann, F. H.; Johnson, N. M. (1972). «Acid rain». Environment. 14 (2): 33–40. doi:10.1080/00139157.1972.9933001.
- ^ Brøgger, Waldemar Christofer (1881). «Note on a contaminated snowfall under the heading Mindre meddelelser (Short communications)». Naturen. 5: 47.
- ^ Ottar, Brynjulf (1976). Dochinger, Leon; Seliga, Thomas (eds.). «Organization of long-range transport of air pollution monitoring in Europe». Proceedings of the First International Symposium on Acid Precipitation and the Forest Ecosystem, May 12–15, 1975, Columbus, Ohio. Upper Darby, PA: USDA Forest Service. 6 (2–4): 105. Bibcode:1976WASP….6..219O. doi:10.1007/BF00182866. S2CID 97680751.
Large amounts of sulphuric acid can be transported over distances up to a few thousand kilometers.
- ^ Odén, Svante (1968). «The Acidification of Air and Precipitation and its Consequences for the Natural Environment». Ecology Committee, Bul. 1. Nat. Sci. Res. Council of Sweden. Retrieved December 5, 2021.
- ^ Satake, Kenichi, ed. (December 6, 2012). Acid Rain 2000 Proceedings from the 6th International Conference on Acidic Deposition: Looking Back to the Past and Thinking of the Future, Tsukuba, Japan, 10–16 December 2000. Netherlands: Springer. p. 20. ISBN 9789400708105. Retrieved December 5, 2021.
Extensive scientific attention to acid deposition arguably began in 1968 when Svante Odén published his landmark paper on acidification (Oden, 1968).
- ^ Hannigan, John A. (1995). Environmental Sociology: A Social Constructionist Perspective. Routledge. p. 130. ISBN 9780415112543. Retrieved December 5, 2021.
Of more immediate impact was the work of Svante Odén, a Swedish soil scientist. Odén, now widely regarded as the ‘father of acid rain studies’ (Park, 1987:6) not only found that the acidity levels of precipitation were increasing in Scandinavia but he was the first to definitively link source and receptor areas.
- ^ «Art Under Wraps Archived August 17, 2014, at the Wayback Machine», Harvard Magazine, March–April 2000
- ^ Likens, G. E.; Bormann, F. H. (1974). «Acid Rain: A Serious Regional Environmental Problem». Science. 184 (4142): 1176–9. Bibcode:1974Sci…184.1176L. doi:10.1126/science.184.4142.1176. PMID 17756304. S2CID 24124373.
- ^ Keller, C. K.; White, T. M.; O’Brien, R.; Smith, J. L. (2006). «Soil CO2 dynamics and fluxes as affected by tree harvest in an experimental sand ecosystem». Journal of Geophysical Research. 111 (G3): G03011. Bibcode:2006JGRG..111.3011K. doi:10.1029/2005JG000157.
- ^ Likens, Gene E.; Bormann, F. Herbert; Johnson, Noye M. (1972). «Acid Rain». Environment: Science and Policy for Sustainable Development. 14 (2): 33–40. doi:10.1080/00139157.1972.9933001.
- ^ Johnson, Noye M.; Driscoll, Charles T.; Eaton, John S.; Likens, Gene E.; McDowell, William H. (September 1, 1981). «‘Acid rain’, dissolved aluminum and chemical weathering at the Hubbard Brook Experimental Forest, New Hampshire». Geochimica et Cosmochimica Acta. 45 (9): 1421–1437. Bibcode:1981GeCoA..45.1421J. doi:10.1016/0016-7037(81)90276-3.
- ^ Hall, Ronald J.; Likens, Gene E.; Fiance, Sandy B.; Hendrey, George R. (August 1, 1980). «Experimental Acidification of a Stream in the Hubbard Brook Experimental Forest, New Hampshire». Ecology. 61 (4): 976–989. doi:10.2307/1936765. ISSN 1939-9170. JSTOR 1936765.
- ^ a b Lackey, R.T. (1997). «Science, policy, and acid rain: lessons learned» (PDF). Renewable Resources Journal. 15 (1): 9–13. Archived (PDF) from the original on May 6, 2013. Retrieved December 15, 2011.
- ^ Winstanley, Derek; Lackey, Robert T.; Warnick, Walter L.; Malanchuk, John (1998). «Acid rain: Science and policy making». Environmental Science & Policy. 1: 51. doi:10.1016/S1462-9011(98)00006-9.
- ^ Times, Robert Reinhold, Special To The New York (June 8, 1982). «ACID RAIN ISSUE CREATES STRESS BETWEEN ADMINISTRATION AND SCIENCE ACADEMY». The New York Times. ISSN 0362-4331. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ «Ronald Reagan on Environment». www.ontheissues.org. Archived from the original on November 25, 2016. Retrieved November 16, 2016.
- ^ «HYSTERIA ABOUT ACID RAIN Even Ronald Reagan now casts it as the villain. He is overriding a lot of scientific evidence. — April 14, 1986». archive.fortune.com. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ «Ronald Reagan: Nomination of William A. Nierenberg To Be a Member of the National Science Board». www.presidency.ucsb.edu. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ «Report of the Acid Rain Peer Review Panel». Document Display | NEPIS | US EPA. July 1984. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ «From tobacco to climate change, ‘merchants of doubt’ undermined the science». Grist. April 17, 2010. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ Franklin, Ben A. (August 18, 1984). «LEGISLATORS SAT WHITE HOUSE SUPPRESSED ACID RAIN REPORT». The New York Times. ISSN 0362-4331. Archived from the original on November 16, 2016. Retrieved November 16, 2016.
- ^ The US National Acid Precipitation Assessment Program : 1990 integrated assessment report. Washington, D.C. : National Acid Precipitation Assessment Program, Office of the Director, [1991]
- ^ «Clean Air Act Title IV — Subchapter A: Acid Deposition Control | Overview of the Clean Air Act and Air Pollution | US EPA». Epa.gov. June 3, 2015. Archived from the original on December 26, 2017. Retrieved March 20, 2018.
- ^ a b John Bachmann, David Calkins, Margo Oge. «Cleaning the Air We Breathe: A Half Century of Progress.» Archived July 6, 2018, at the Wayback Machine EPA Alumni Association. September 2017. Pages 26-27.
- ^ «US EPA: A Brief History of Acid Rain». United States Environmental Protection Agency. 2002. Archived from the original on September 25, 2010. Retrieved November 18, 2010.
- ^ a b ‘Cap-and-trade’ model eyed for cutting greenhouse gases Archived March 16, 2012, at the Wayback Machine, San Francisco Chronicle, December 3, 2007.
- ^ «Facts On File News Services Databases». 2facts.com. Retrieved November 18, 2010.[permanent dead link]
- ^ Gilberston, T. and Reyes, O. 2009. Carbon Trading: how it works and why it fails Archived January 6, 2010, at the Wayback Machine. Dag Hammarskjöld Foundation: 22
- ^ Acid Rain Program 2007 Progress Report Archived May 1, 2011, at the Wayback Machine, United States Environmental Protection Agency, January 2009.
- ^ Gerdes, Justin. «Cap and Trade Curbed Acid Rain: 7 Reasons Why It Can Do The Same For Climate Change». Forbes. Retrieved October 27, 2014.
- ^ a b Muki Haklay (2015). «Citizen Science and Policy: A European Perspective» (PDF). Woodrow Wilson International Center for Scholars. p. 11. Archived from the original (PDF) on October 18, 2016. Retrieved June 3, 2016.
- ^ a b R. Kerson (1989). «Lab for the Environment». MIT Technology Review. Vol. 92, no. 1. pp. 11–12.
- ^ Albin, Tom; Paulsen, Steve (1985). «5: Environmental and Economic Interests in Canada and the United States». In Schmandt, Jurgen; Roderick, Hilliard (eds.). Acid Rain and Friendly Neighbors: The Policy Dispute Between Canada and the United States. Duke University Press. p. 129. ISBN 9780822308706. Retrieved December 5, 2021.
- ^ a b «IISD Experimental Lakes Area: The world’s living freshwater laboratory». BioLab Business Magazine. February 12, 2020. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- ^ a b Luoma, Jon R. (September 13, 1988). «Bold Experiment in Lakes Tracks the Relentless Toll of Acid Rain». The New York Times. ISSN 0362-4331. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- ^ «A Canadian Scientist Explains How Acid Rain is Still Making its Mark». IISD Experimental Lakes Area. May 16, 2018. Archived from the original on July 6, 2020. Retrieved July 6, 2020.
- ^ a b c Canada, Environment and Climate Change (June 3, 2004). «Acid rain history». aem. Archived from the original on July 7, 2020. Retrieved July 6, 2020.
- ^ Berresheim, H.; Wine, P.H. and Davies D.D. (1995). «Sulfur in the Atmosphere». In Composition, Chemistry and Climate of the Atmosphere, ed. H.B. Singh. Van Nostrand Rheingold ISBN 0-442-01264-0
- ^ Nemecek, T.; Poore, J. (June 1, 2018). «Reducing food’s environmental impacts through producers and consumers». Science. 360 (6392): 987–992. Bibcode:2018Sci…360..987P. doi:10.1126/science.aaq0216. ISSN 0036-8075. PMID 29853680.
- ^ Floor, G. H.; Calabrese, S.; Román-Ross, G.; D’Alessandro, W.; Aiuppa, A. (October 23, 2011). «Selenium mobilization in soils due to volcanic derived acid rain: An example from Mt Etna volcano, Sicily». Chemical Geology. 289 (3): 235–244. Bibcode:2011ChGeo.289..235F. doi:10.1016/j.chemgeo.2011.08.004. hdl:10447/66526. ISSN 0009-2541. S2CID 140741081. Archived from the original on January 24, 2012. Retrieved April 22, 2020.
- ^ «Acid Rain: Causes, Effects and Solutions». Live Science. July 14, 2018. Archived from the original on August 23, 2019. Retrieved August 23, 2019.
- ^ a b Likens, G. E.; Wright, R. F.; Galloway, J. N.; Butler, T. J. (1979). «Acid rain». Scientific American. 241 (4): 43–51. Bibcode:1979SciAm.241d..43L. doi:10.1038/scientificamerican1079-43.
- ^ Galloway, JN; Dianwu, Z; Jiling, X; Likens, GE (1987). «Acid rain: China, United States, and a remote area». Science. 236 (4808): 1559–62. Bibcode:1987Sci…236.1559G. doi:10.1126/science.236.4808.1559. PMID 17835740. S2CID 39308177.
- ^ Chandru (September 9, 2006). «CHINA: Industrialization pollutes its country side with Acid Rain». Southasiaanalysis.org. Archived from the original on June 20, 2010. Retrieved November 18, 2010.
- ^ Lefohn, A.S.; Husar, J.D.; Husar, R.B. (1999), Global Sulfur Emissions Database, United States: A.S.L. & Associates, archived from the original on June 6, 2013, retrieved February 16, 2013
- ^ Likens, G. E. (1984). «Acid rain: the smokestack is the «smoking gun»«. Garden. 8 (4): 12–18.
- ^ Clean Air Act Reduces Acid Rain In Eastern United States Archived August 8, 2018, at the Wayback Machine, ScienceDaily, September 28, 1998
- ^ a b US EPA, OAR (February 9, 2016). «What is Acid Rain?». US EPA. Archived from the original on May 23, 2020. Retrieved April 14, 2020.
- ^ «UK National Air Quality Archive: Air Pollution Glossary». Airquality.co.uk. April 1, 2002. Archived from the original on April 17, 2009. Retrieved November 18, 2010.
- ^ a b «Effects of Acid Rain — Surface Waters and Aquatic Animals». US EPA. Archived from the original on May 14, 2009.
- ^ Kesler, Stephen (2015). Mineral Resources, Economics and the Environment. Cambridge University. ISBN 9781107074910.
- ^ Rodhe, Henning; Dentener, Frank; Schulz, Michael (October 1, 2002). «The Global Distribution of Acidifying Wet Deposition». Environmental Science & Technology. 36 (20): 4382–4388. Bibcode:2002EnST…36.4382R. doi:10.1021/es020057g. ISSN 0013-936X. PMID 12387412.
- ^ Likens, G. E.; Driscoll, C. T.; Buso, D. C. (1996). «Long-Term Effects of Acid Rain: Response and Recovery of a Forest Ecosystem» (PDF). Science. 272 (5259): 244. Bibcode:1996Sci…272..244L. doi:10.1126/science.272.5259.244. S2CID 178546205. Archived (PDF) from the original on December 24, 2012. Retrieved February 9, 2013.
- ^
- ^ Evans, Lance S.; Gmur, Nicholas F.; Costa, Filomena Da (1977). «Leaf Surface and Histological Perturbations of Leaves of Phaseolus Vulgaris and Helianthus Annuus After Exposure to Simulated Acid Rain». American Journal of Botany. 64 (7): 903–913. doi:10.1002/j.1537-2197.1977.tb11934.x. ISSN 1537-2197.
- ^ Du, Yan-Jun; Wei, Ming-Li; Reddy, Krishna R.; Liu, Zhao-Peng; Jin, Fei (April 30, 2014). «Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil». Journal of Hazardous Materials. 271: 131–140. doi:10.1016/j.jhazmat.2014.02.002. ISSN 0304-3894. PMID 24637445. Archived from the original on February 21, 2021. Retrieved April 22, 2020.
- ^ Sun, Jingwen; Hu, Huiqing; Li, Yueli; Wang, Lihong; Zhou, Qing; Huang, Xiaohua (September 1, 2016). «Effects and mechanism of acid rain on plant chloroplast ATP synthase» (PDF). Environmental Science and Pollution Research. 23 (18): 18296–18306. doi:10.1007/s11356-016-7016-3. ISSN 1614-7499. PMID 27278067. S2CID 22862843. Archived (PDF) from the original on October 9, 2022.
- ^ Stoyanova, D.; Velikova, V. (December 1, 1997). «Effects of Simulated Acid Rain on Chloroplast Ultrastructure of Primary Leaves of Phaseolus Vulgaris» (PDF). Biologia Plantarum. 40 (4): 589–595. doi:10.1023/A:1001761421851. ISSN 1573-8264. S2CID 20728684. Archived (PDF) from the original on October 9, 2022.
- ^ Johnson, Dale W.; Turner, John; Kelly, J. M. (1982). «The effects of acid rain on forest nutrient status». Water Resources Research. 18 (3): 449–461. Bibcode:1982WRR….18..449J. doi:10.1029/WR018i003p00449. ISSN 1944-7973.
- ^ DeHayes, D.H., Schaberg, P.G. and G.R. Strimbeck. (2001). Red Spruce Hardiness and Freezing Injury Susceptibility. In: F. Bigras, ed. Conifer Cold Hardiness. Kluwer Academic Publishers, the Netherlands ISBN 0-7923-6636-0.
- ^ Lazarus, Brynne E.; Schaberg, Paul G.; Hawley, Gary J.; DeHayes, Donald H. (2006). «Landscape-scale spatial patterns of winter injury to red spruce foliage in a year of heavy region-wide injury» (PDF). Can. J. For. Res. 36: 142–152. doi:10.1139/x05-236. highbeam copy
- ^ a b «Acid Rain Has A Disproportionate Impact On Coastal Waters». ScienceDaily. Archived from the original on June 26, 2020. Retrieved June 26, 2020.
- ^ «Acid Rain Has Disproportionate Impact on Near-Shore Ocean Waters — Windows to the Universe». www.windows2universe.org. Archived from the original on February 28, 2017. Retrieved February 27, 2017.
- ^ Reisener, A.; Stäckle, B.; Snethlage, R. (1995). «ICP on effects on materials». Water, Air, & Soil Pollution. 85 (4): 2701–2706. Bibcode:1995WASP…85.2701R. doi:10.1007/BF01186242. S2CID 94721996.
- ^ «Approaches in modeling the impact of air pollution-induced material degradation» (PDF). Archived from the original (PDF) on July 16, 2011. Retrieved November 18, 2010.
- ^ Ed. Hatier (1993). «Acid Rain in Europe». United Nations Environment Programme GRID Arendal. Archived from the original on August 22, 2009. Retrieved January 31, 2010.
- ^ US Environmental Protection Agency (2008). «Clean Air Markets 2008 Highlights». Retrieved January 31, 2010.
- ^ «Acid Rain — Green Education Foundation | GEF | Sustainability Education». www.greeneducationfoundation.org. Archived from the original on October 21, 2017. Retrieved November 2, 2017.
- ^ «The Convention and its achievements | UNECE». unece.org. Retrieved October 22, 2021.
- ^ Moses, Elizabeth; Cardenas, Beatriz; Seddon, Jessica (February 25, 2020). «The Most Successful Air Pollution Treaty You’ve Never Heard Of».
- ^ «International Agreements on Acid Rain». www.enviropedia.org.uk. Archived from the original on October 22, 2021. Retrieved October 22, 2021.
- ^ «Talks start to form network to monitor Asia’s acid rain». The Japan Times. October 26, 2000. Retrieved October 22, 2021.
- ^ Totsuka, T., Sase, H., Shimizu, H. (2005). Major activities of acid deposition monitoring network in East Asia (EANET) and related studies. In: Omasa, K., Nouchi, I., De Kok, L.J. (eds) Plant Responses to Air Pollution and Global Change. Springer, Tokyo. https://doi.org/10.1007/4-431-31014-2_28
- ^ «EANET National Focal Points» https://www.eanet.asia/about/national-focal-points/ retrieved February, 16 2023.
- ^ Former Deputy Administrator Hank Habicht talks about management at EPA. An Interview with Hank Habicht Video, Transcript Archived April 12, 2019, at the Wayback Machine (see p6). December 21, 2012.
- ^ Clean Air Act Amendments of 1990, 42 U.S. Code 7651 Archived March 28, 2021, at the Wayback Machine
External links
Wikimedia Commons has media related to Acid rain.
- National Acid Precipitation Assessment Program Report – a 98-page report to Congress (2005)
- Acid rain for schools
- Acid rain for schools – Hubbard Brook
- United States Environmental Protection Agency – New England Acid Rain Program (superficial)
- Acid Rain (more depth than ref. above)
- U.S. Geological Survey – What is acid rain?
- Acid Rain: A Continuing National Tragedy – a report from The Adirondack Council on acid rain in the Adirondack region (1998)
- What Happens to Acid Rain?
- Acid Rain and how it affects fish and other aquatic organisms
- Fourth Report for Policy Makers (RPM4): Towards Clean Air for Sustainable Future in East Asia through Collaborative Activities- a report for policy-makers, Acid Deposition Monitoring Network in East Asia, EANET, (2019).
What Is The Meaning Of Acid Rain?
The Meaning Of Acid Rain In English,
The meaning of acid rain is that it is a rainfall made so acidic by atmospheric pollution that it causes environmental harm, especially to forests and lakes. The main cause the main reason behind the acid rain is the industrial burning of coal and other fossil fuels, the waste gases from industries which contain sulphur and nitrogen oxide which combine with atmospheric water to form acids.
The acid clouds grow on sulphur dioxide emissions from refineries. Acid rain can also be defined as the rain or any other form of precipitation that is usually acidic because it has elevated levels of hydrogen ions, which means it has the pH very low than the normal pH level or value.
The acid rain has very harmful effects on plants, aquatic animals and infrastructure as well as on human beings. The sulphur dioxide and nitrogen oxides are eliminated by the industries and then react with the water molecules in the atmosphere to produce the acids, which results in causing acid rains.
Acid rains have also been shown to have the worst influence on forests, freshwaters, and soils, destroying the insect and aquatic life forms, causing the paint to peel, undermining of steel structures namely bridges, and weathering of stone buildings and statues as well as having a bad influence on human health.
Click here – What Is Meaning Of Acknowledgement? Find Out Meaning Of Acknowledgement.
What Are The Synonyms Of Acid Rain?
Synonyms Of Acid Rain Are:
- Not Available The Synonyms Of Acid Rain.
What Are The Antonyms Of Acid Rain?
Antonyms Of Acid Rain Are:
- Not Available The Antonyms Of Acid Rain.
Related Words Of Acid Rain Are:
- Not Available The Related Words Of Acid Rain.
What Is The Noun Form Of Acid Rain?
Noun Form Of Acid Rain Is:
- Acid Rain
What Is The Verb Form Of Acid Rain?
Verb Form Of Acid Rain Is:
- Not Available The Verb Form Of Acid Rain.
What Is The Adjective Of Acid Rain?
Adjective Of Acid Rain Is:
- Not Available The Adjective Of Acid Rain.
Examples Of Using The Word Acid Rain Are:
- When acid rain falls in lakes and rivers, it lifts up the acidity of the water and can also kill or seriously damage aquatic organisms.
- Coal is the most polluting fuel in our energy mix donating, among other things, to smog, acid rain, and global warming.
- Nitrogen oxides can react with hydrocarbons to produce nitric acid, a primary constituent in acid rain.
- Unlike burning coal or oil, wave and wind machines do not emit sulphur and cause acid rain.
- One industrial application of acid-base titration is in the try to control the damage from acid rain and connected air pollution.
- The power created from fossil fuels causes smog, mercury pollution, acid rain, and global warming.
- The city children got an opportunity to be in the country and become attentive to the watershed system, acid rain, and pollution.
- Wind energy doesn’t create sulfur dioxide or nitrous oxides that cause acid rain, and it does not damage the Earth’s climate.
- Global environmental problems like acid rain, ozone depletion, and climate change are relatively current phenomena.
- Innumerable lakes have been more or less disinfected by acid rain.
- This can be sourced by anything such as acid rain, pollution, sheep dip getting in, anything.
FAQ
What Is Acid Rain In Sentence?
Acid rain caused by our coal-fired power stations does immense harm to the environment.
Why Acid Rain Is Called?
Acid rain is caused by a chemical reaction that begins when compounds like sulfur dioxide and nitrogen oxides are released into the air. These substances can rise very high into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants, known as acid rain.
What Is Acid Rain And Why Is It Bad?
Acid rain leaches aluminum from the soil. That aluminum may be harmful to plants as well as animals. Acid rain also removes minerals and nutrients from the soil that trees need to grow.
Can Acid Rain Hurt You?
The harm comes from breathing in particles from acid rain. If you’re exposed to high concentrations of nitric and sulfuric acid — especially over time — it can cause these problems: Irritation to eyes, skin, and mucous membranes can come from contact with one or both acids.
You were searching for the meaning of Acid Rain
I hope you had got the meaning of Acid Rain with synonyms and antonyms.
I Hope All Queries Covered In This Post LIke
What is the Meaning of Acid Rain In English?
What is meant By Acid Rain?
What Does Acid Rain mean?
Click here – What Is The Meaning Of Aclamaciones? Find Out Meaning Of Aclamacionies.
Meaning Acid Rain
What does Acid Rain mean? Here you find 86 meanings of the word Acid Rain. You can also add a definition of Acid Rain yourself
1 |
0 Rain or precipitation that contains elevated levels of hydrogen ions. Elevated hydrogen levels decrease pH levels. Decreased pH level creates an acidic atmosphere. Acid rain results from the combination of fossil fuel emissions and water in the atmosphere. The environmental effects of acid rain include the acidification of lakes and streams, damage [..]
|
2 |
0 Acid RainRain with a pH less than 5.6. Normal pH of precipitation is 5.6.
|
3 |
0 Acid RainAlso called acid precipitation or acid deposition, acid rain is precipitation containing harmful amounts of nitric and sulfuric acids formed primarily by sulfur dioxide and nitrogen oxides release [..]
|
4 |
0 Acid RainRain containing relatively high concentrations of acid-forming chemicals that have been released into the atmosphere and combined with water vapor; harmful to the environment.
|
5 |
0 Acid RainPluie acide
|
6 |
0 Acid RainThe acidity in rain due to gases from internal combustion engines and coal- and oil-burning power plants.
|
7 |
0 Acid Rainprecipitation with high levels of nitric and sulfuric acids. Acid rain can be manmade or occur naturally.
|
8 |
0 Acid RainA popular expression for the deposition by rainfall of various airborne pollutants (especially SO2 and NO2) that have harmful effects on vegetation, soils, buildings and other external structures.
|
9 |
0 Acid RainFossil fuels can release chemicals such as sulphur when they are burnt (as petrol is in a car, or coal is in a power station). These chemicals can dissolve in atmospheric water and make rainfall unnat [..]
|
10 |
0 Acid Rainprecipitation heavy with nitric and sulfuric acid. Most of it is generated by sulfur dioxide and nitrogen dioxide (air pollution). Its pH is less than 5.6. Results include fish and plant deaths, corrosion, groundwater pollution, and soil erosion. Its long-term effects are unknown.
|
11 |
0 Acid Rainrefers to the unnatural increase, through human pollution, in the acidity of water precipitation. Most commonly sulphuric and nitric acids formed from by-products of fossil-fuel burning and metal smel [..]
|
12 |
0 Acid RainAbnormally acidic rainfall, most often containing dilute concentrations of sulfuric acid or nitric acid.
|
13 |
0 Acid RainThe combination of sulfur dioxide and nitrogen oxides with water in the atmosphere. This combination produces acidicprecipitation called acid rain. The burning of fossil fuels is a major contributor to acid rain. acid
|
14 |
0 Acid RainRain that has a flamboyantly low pH, due to contact with atmospheric pollutants such as sulphuric oxides.
|
15 |
0 Acid RainAcid rain is polluted and harmful to the environment. Acid rain has a low pH. Acid rain may have been a component of the K-T extinction.
|
16 |
0 Acid RainRain which is unusually acidic (pH of less than the natural range of 5 to 6), caused mainly by atmospheric pollution with sulphur dioxide and nitrogen compounds.
|
17 |
0 Acid RainPrecipitation that has become acidic (low pH) due to the emission of sulfur oxides from fossil fuel-burning power plants. Source: U.S. Department of Energy / Office of Energy Efficiency and Renewable Energy
|
18 |
0 Acid RainRain that has a relatively low pH (i.e., relatively high acidity) because of air pollution.
|
19 |
0 Acid Rain[noun] Rain with a pH less than 5.
|
20 |
0 Acid Rain
|
21 |
0 Acid Rainrain that has become more acidic than normal due to pollution. activation energy —
|
22 |
0 Acid RainRain (and snow, fog, dust particles, etc.) containing acids that form in the atmosphere when sulfur dioxides and nitrogen oxides from industrial emissions and automobile exhaust combine with water.
|
23 |
0 Acid RainRain that is acidic due to dissolved gases, such as sulphur dioxide, in the atmosphere. These dissolved gases are present because of the burning of fossil fuels.
|
24 |
0 Acid RainAcid rain or acid deposition is a form of precipitation (rain, snow, sleet, or hail) containing high levels of sulfuric or nitric acids ( p H below 5.5-5.6). Acid rain is produced when sulfur dioxide [..]
|
25 |
0 Acid RainRain or any other type of precipitation that is abnormally acidic as a result of air pollution (Lesson 14)
|
26 |
0 Acid RainCloud or rain droplets containing pollutants, such as oxides of sulfur and nitrogen, to make them acidic (eg. pH < 5.6).
|
27 |
0 Acid RainAlso called acid precipitation or acid deposition, acid rain is precipitation containing harmful amounts of nitric and sulfuric acids formed primarily by nitrogen oxides and sulfur oxides released into the atmosphere when fossil fuels are burned. It can be wet precipitation (rain, snow, or fog) or dry precipitation (absorbed gaseous and particula [..]
|
28 |
0 Acid RainRain that has become acidic due to the emission of sulfur dioxide and nitrogen oxides.
|
29 |
0 Acid RainRain that has become acidic due to the emission of sulfur dioxide and nitrogen oxides.
|
30 |
0 Acid RainA term generally used to describe rain (and snow) with acidity lower than pH 5.6 (7 is neutral – the lower the number, the greater the amount of acid – normal rain is about 5.8). The acidity of precipitation in the United States due to acidic gases, other than carbon dioxide, is about 60 percent sulfuric acid, 30 percent nitric acid, and 10 per [..]
|
31 |
0 Acid RainForms when pollution is combined with water in the atmosphere. When sulfur dioxide and nitrogen oxid…
|
32 |
0 Acid RainAcid rain is a generic term used for precipitation that contains an abnormally high concentration of sulfuric and nitric acid. These acids form in the atmosphere when industrial gas emissions combine [..]
|
33 |
0 Acid RainAir pollution produced when acid chemicals such as sulfur oxides and nitrogen oxides combine with moisture in the air and fall to the Earth as acidic rain, snow, fog, or mist. The main sources of these pollutants are vehicles and industrial and power-generating plants.
|
34 |
0 Acid RainThe deposition of acids (sulphuric and nitric) in rain. An environmental problem resulting from certain industrial activities and the burning of petroleum based fuels (trafic exhaust gases).
|
35 |
0 Acid RainThe precipitation of dilute solutions of strong mineral acids, formed by the mixing in the atmosphere of various industrial pollutants — primarily sulfur dioxide and nitrogen oxides — with naturally [..]
|
36 |
0 Acid RainPrecipitation which has been rendered (made) acidic by airborne pollutants.
|
37 |
0 Acid Rain(See: acid deposition.)
|
38 |
0 Acid Rain(See: acid deposition.)
|
39 |
0 Acid RainThe deposition of airborne acids by rain or snow great distances from where these substances are discharged into the atmosphere by the burning of fossil fuels. Acid rain adversely affects aquatic and terrestrial environments.
|
40 |
0 Acid Rainis rain mixed mainly with nitric and sulphuric acid, that arise from emissions released during the burning of fossil fuels.
|
41 |
0 Acid RainPrecipitation which has a pH of less than 5.6.
|
42 |
0 Acid RainAcidic Water usually pH 2.5 to 4.5, which Poisons the Ecosystem and adversely Affects Plants, Fishes, and Mammals. It is caused by industrial pollutants, mainly Sulfur Oxides and Nitrogen Oxides, emit [..]
|
43 |
0 Acid RainAcidic water usually pH 2.5 to 4.5, which poisons the ecosystem and adversely affects plants, fishes, and mammals. It is caused by industrial pollutants, mainly sulfur oxides and nitrogen oxides, emit [..]
|
44 |
0 Acid RainAcids form when certain atmospheric gases (primarily carbon dioxide, sulfur dioxide, and nitrogen oxides) come in contact with water in the atmosphere or on the ground and are chemically converted to [..]
|
45 |
0 Acid RainPrecipitation that has been rendered (made) acidic by airborne pollutants.
|
46 |
0 Acid RainRain with pH below 5.5.
|
47 |
0 Acid RainNatural rainfall that contains nitric and sulfuric acids due to nitrogen oxide (NOx) and sulfur dioxide discharged into the air by industries, power plants, automobiles and other emission sources.
|
48 |
0 Acid RainAcid Rain is rain that has been made acidic by certain pollutants released in the air, most commonly caused by human activities. Acid rain creates tiny particles which can easily enter people’s l [..]
|
49 |
0 Acid Rainrain having a pH lower than 5.6 (the pH of natural rainwater), usually caused by sulfuric acid and/or nitric acid from air pollution.
|
50 |
0 Acid RainRain that is especially acidic (pH is less than 5.2). Principal components of acid rain typically include nitric and sulfuric acid. These may be formed by the combination of nitrogen and sulfur oxides [..]
|
51 |
0 Acid RainWhen atmospheric pollutants such as sulphur dioxide and nitrogen oxides mix with water vapour in the air, they are converted to sulphuric and nitric acids. These acids make the rain acidic, hence the [..]
|
52 |
0 Acid RainCloud or rain droplets containing pollutants, such as oxides of sulfur and nitrogen, to make them acidic.
|
53 |
0 Acid RainAir pollution produced when acid chemicals are incorporated into rain, snow, fog or mist. The “acid” in acid rain comes from sulfur oxides and nitrogen oxides, products of burning coal and other fuels and from certain industrial processes. The sulfur oxides and nitrogen oxides are related to two strong acids: sulfuric acid and nitric acid. When [..]
|
54 |
0 Acid RainAcidified particulate matter in the atmosphere that is deposited by precipitation onto a surface, often eroding the surface away. This precipitation generally has a pH less than 5 and sometimes much l [..]
|
55 |
0 Acid Rainfalling rain
|
56 |
0 Acid Rain<biology, plant biology> This is rain which has turned acidic because of the presence of sulphur or nitrogen oxides (both created from burning coal and other fossil fuels) in the atmosphere. Acid rain is a serious environmental problem, it can kill trees and harm plants and animals in lakes and ponds. (09 Oct 1997)
|
57 |
0 Acid RainAir pollution produced when acid chemicals are incorporated into rain, snow, fog or mist. The "acid" in acid rain comes from sulfur oxides and nitrogen oxides, products of burning co [..]
|
58 |
0 Acid RainRain containing relatively high concentrations of acid-forming chemicals that have been released into the atmosphere and combined with water vapor; harmful to the environment.
|
59 |
0 Acid RainAcid rain forms when the pollutants sulphur dioxide (SO2) and nitrogen oxides (NOx) mix with water to form acids that fall as precipitation (fog, rain or snow). This is referred to as «wet deposi [..]
|
60 |
0 Acid RainSee Acidification
|
61 |
0 Acid RainForms of precipitation (such as rain, snow or sleet) containing high levels of sulphuric or nitric acids (with pH levels below 5.5–5.6). Also includes dry deposited gases and particles that fall bac [..]
|
62 |
0 Acid Rainthe acidic rainfall which results when rain combines with sulfur oxides emissions from combustion of fossil fuels.
|
63 |
0 Acid RainPrecipitation that becomes acidic due to acid-forming precursors put into the atmosphere by human activities.
|
64 |
0 Acid Rainrain with a pH of less than 5.6; results from atmospheric moisture mixing with sulphur and nitrogen oxides emitted from burning fossil fuels or from volcanic activity; may cause damage to buildings, monuments, car finishes, crops, forests, wildlife habitats, and aquatic life.
|
65 |
0 Acid RainAlso called acid precipitation or acid deposition, acid rain is precipitation containing harmful amounts of nitric and sulfuric acids formed primarily by nitrogen oxides and sulfur oxides released into the atmosphere when fossil fuels are burned. It can be wet precipitation (rain, snow or fog) or dry precipitation (absorbed gaseous and particulate [..]
|
66 |
0 Acid RainCloud or rain droplets combine with gaseous pollutants, such as oxides of sulfur and nitrogen, to make falling rain or snow acidic.
|
67 |
0 Acid RainRain that is more acidic than normal because water vapour has condensed ion to particles of sulphate or nitrogen oxide.
|
68 |
0 Acid RainCloud
|
69 |
0 Acid Rainrain or other forms of precipitation that is unusually acidic.
|
70 |
0 Acid RainGenerally , precipitation in any form, or dry deposition , with a pH lower than would be expected from natural and artificial causes .
|
71 |
0 Acid RainThe precipitation of dilute solutions of strong mineral acids, formed by the mixing in the atmosphere of various industrial pollutants — primarily sulfur dioxide and nitrogen oxides — with naturally [..]
|
72 |
0 Acid RainAny form of precipitation (rain, snow, hail or fog) whose acidity has been increased through the uptake of acid pollutants from the air.
|
73 |
0 Acid RainA complex chemical and atmospheric phenomenon that occurs when emissions of sulfur and nitrogen compounds and other substances are transformed by chemical processes in the atmosphere, often far from t [..]
|
74 |
0 Acid Rainprecipitation, whether rain or snow, where the water has an acidity greater than normal (effectively a pH of less than 5.7). It derives from interaction of water vapour in air with sulfur and nitrog [..]
|
75 |
0 Acid RainCloud or rain droplets containing pollutants, such as oxides of sulfur and nitrogen, to make them acidic.
|
76 |
0 Acid RainIs caused by emissions of sulphur dioxide and nitrogen oxides. Although natural sources of sulphur oxides and nitrogen oxides do exist, more than 90% of the sulphur and 95% of the nitrogen emissions o [..]
|
77 |
0 Acid RainPrecipitation that carries to earth sulfuric and nitric acid accumulated from air pollutants.
|
78 |
0 Acid RainAcid rain is a generic term used for precipitation that contains an abnormally high concentration of Sulfuric and Nitric acid. These acids form in the Atmosphere when industrial gas emissions combine with water, and have negative impacts on the environment and human health.
|
79 |
0 Acid RainRain containing relatively high concentrations of acid-forming chemicals that have been released into the atmosphere and combined with water vapor; harmful to the environment.
|
80 |
0 Acid RainAtmospheric precipitation with an pH below 5.6 to 5.7.
|
81 |
0 Acid RainPrecipitation that possesses elevated levels of nitric and sulphuric acids which can have harmful effects on vegetation, aquatic habitats, human health and infrastructure.
|
82 |
0 Acid RainA washout of an excessive concentration of acidic compounds in the atmosphere, resulting from chemical pollutants such as sulphur and nitrogen compounds. When deposited these increase the acidity of t [..]
|
83 |
0 Acid RainThe wet deposition only of acidifying substances from the atmosphere. See also acidifying deposition.
|
84 |
0 Acid RainThe increased acidity of rainfall which is caused by emissions of sulfur dioxide and nitrogen oxides from power plants and automobiles.
|
85 |
0 Acid RainPrecipitation which has been rendered (made) acidic by airborne pollutants; Also see Acid deposition
|
86 |
0 Acid RainRain (and snow, fog, dust particles, etc.) containing acids that form in the atmosphere when sulfur dioxides and nitrogen oxides from industrial emissions and automobile exhaust combine with water.
|
Dictionary.university is a dictionary written by people like you and me.
Please help and add a word. All sort of words are welcome!
Add meaning
- acid rain
-
кислотный дождь
Англо-русский современный словарь.
2014.
Смотреть что такое «acid rain» в других словарях:
-
Acid rain — is rain or any other form of precipitation that is unusually acidic. It has harmful effects on plants, aquatic animals, and infastructure. Acid rain is mostly caused by human emissions of sulfur and nitrogen compounds which react in the… … Wikipedia
-
Acid Rain — Лучшие хиты Esham Дата выпу … Википедия
-
acid rain — n acid precipitation esp. in the form of rain … Medical dictionary
-
acid rain — n [U] rain that contains harmful acid which can damage the environment and is caused by chemicals in the air, for example from cars or factories … Dictionary of contemporary English
-
acid rain — noun uncount rain containing a high level of acid that can damage the environment. It is caused by pollution in the air … Usage of the words and phrases in modern English
-
acid rain — n. rain or other precipitation with a high concentration of acids produced by sulfur dioxide, nitrogen dioxide, and other such gases that result from the combustion of fossil fuels: it has a destructive effect on plant and aquatic life, buildings … English World dictionary
-
Acid rain — Acid rain. См. Кислотный дождь. (Источник: «Металлы и сплавы. Справочник.» Под редакцией Ю.П. Солнцева; НПО Профессионал , НПО Мир и семья ; Санкт Петербург, 2003 г.) … Словарь металлургических терминов
-
acid rain — ► NOUN ▪ rainfall made acidic by sulphur and nitrogen oxides from the industrial burning of fossil fuels … English terms dictionary
-
acid rain — noun rain containing acids that form in the atmosphere when industrial gas emissions (especially sulfur dioxide and nitrogen oxides) combine with water • Syn: ↑acid precipitation • Hypernyms: ↑air pollution * * * noun [noncount] : rain that… … Useful english dictionary
-
acid rain — precipitation, as rain, snow, or sleet, containing relatively high concentrations of acid forming chemicals, as the pollutants from coal smoke, chemical manufacturing, and smelting, that have been released into the atmosphere and combined with… … Universalium
-
acid rain — /æsəd ˈreɪn/ (say asuhd rayn) noun highly acidic rain, caused by pollution in the atmosphere. Acid rain is formed when airborne pollutants from the burning of fossil fuels, and from industrial activities and vehicles emissions, as well as from… …
: rain that has increased acidity caused by environmental factors (such as atmospheric pollutants)
Example Sentences
trees damaged by acid rain
Recent Examples on the Web
If not treated, the exhaust gases can also contribute to the same acid rain that plagued communist Germany thanks to lignite’s high sulfur content and sundry other impurities including toxic heavy metals.
—
Acidification from carbon dioxide overload in the atmosphere is different than acid rain caused by sulfur dioxide and nitrogen oxides from fossil fuel burning for electric power generation or manufacturing.
—
Flash acid rain, right after warmups—figures.
—
What happened to acid rain?
—
In the mid-2000s, EPA regulations forced many U.S. power plants to invest in upgrading smokestacks with scrubbers that remove nearly all sulfur dioxide — a pollutant that can harm human health and contribute to acid rain.
—
When burned at high temperatures, ammonia produces nitrogen dioxide, which contributes to smog and acid rain and can harm people’s respiratory systems.
—
Releasing a lot of sulfur dioxide has the potential to trigger acid rain, irritate people’s lungs, and even worsen the Antarctic ozone hole.
—
However, Dowty Beech said hydrochloric acid, which is the byproduct of the vinyl chloride that was burned, does not typically get picked up by the air current or create acid rain.
—
See More
These examples are programmatically compiled from various online sources to illustrate current usage of the word ‘acid rain.’ Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
First Known Use
1845, in the meaning defined above
Time Traveler
The first known use of acid rain was
in 1845
Dictionary Entries Near acid rain
Cite this Entry
“Acid rain.” Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/acid%20rain. Accessed 14 Apr. 2023.
Share
More from Merriam-Webster on acid rain
Last Updated:
13 Apr 2023
— Updated example sentences
Subscribe to America’s largest dictionary and get thousands more definitions and advanced search—ad free!
Merriam-Webster unabridged
Acid rain, or acid deposition, is defined or a term that includes any form of precipitation with acidic components, like sulphuric or nitric acid, that falls from the atmosphere in wet or dry forms, including rain, snow, fog, hail and dust.
—
What Causes Acid Rain?
Acid rain is a result of sulphur dioxide (SO2) and nitrogen oxides (NOX) being emitted into the atmosphere and transported by wind and air, making it a problem for all, not just those who live near the sources of the pollution. Acid rain consists of sulphuric and nitric acids, formed when aforementioned gases mix and react with water, oxygen and other chemicals, which is then mix with water again and other materials before falling to the ground.
While a small portion of the SO2 and NOX that cause acid rain comes from natural sources such as volcanoes and rotting vegetation, most of it comes from human activities, like the burning of fossil fuels, through electricity generation, oil refineries and vehicles. Over the past few decades, humans have emitted so many different chemicals that the mix of gases in the atmosphere has been changed.
As stated, acid deposition can occur in wet and dry forms. When wet, it is what we most commonly think of as acid rain. This falls as rain, snow, fog or hail. Dry deposition occurs when acidic particles and gases may deposit on surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can pose a risk to human health. When these accumulated acids are washed off the surface by the next rain, the acidic water flows over and through the ground and could be harmful to plants and wildlife. The amount of acidity in the atmosphere that occurs through dry deposition depends on the amount of rainfall an area receives.
You might also like: What Are Carbon Sinks?
What are the Effects of Acid Rain?
While sulphur dioxides actually have a cooling effect on the atmosphere, nitrogen oxides contribute to the formation of ozone, which can be harmful to people and plants. Both of these gases are causes for concern because they can spread easily via air pollution and acid rain.
Acid rain makes waters more acidic, posing a risk to lakes, streams, wetlands and other aquatic environments. Increasing a body of water’s acidity results in more aluminium absorption from soil, which is then transported to lakes and streams. This makes waters toxic to crayfish, clams, fish and other aquatic animals.
While some species can handle acidic waters better than others, in connected ecosystems, there will be a domino effect throughout the food chain, whereby one species dying off because it cannot tolerate acidic rain will leave another without food, killing that species off as well.
Acid rain and fog also damage forests, especially those at higher elevations. The acid deposits rob the soil of nutrients such as calcium and cause aluminium to be released into the soil, which makes it difficult for trees to suck up water. The results are less healthy trees and planets that are more vulnerable to cold temperatures, insects and disease. Additionally, the trees’ ability to reproduce is compromised.
Physical structures such as limestone buildings and cars can also be affected by acid rain. When it takes the form of fog, it can be inhaled and cause health problems, like eye irritation and asthma.
How Do We Measure It?
Acid rain is measured using a pH scale for which 7.0 is neutral. The lower a substance’s pH (less than 7), the more acidic it is; the higher it is (greater than 7), the more alkaline it is.
Normally, rain has a pH of about 5.6, making it slightly acidic because CO2 dissolves into it, forming a weak carbonic acid. However, normal precipitation reacts with alkaline chemicals that can be found in air, soils, bedrock, lakes and streams, the reactions of which usually neutralise natural acids. Acid rain has a pH of between 4.2 and 4.4.
When acid deposition is washed into lakes and streams, it can cause some to turn acidic.
How Do We End It?
Unfortunately, the only way to reverse the occurrence of acid rain is to curb the release of the pollutants that cause it, meaning that we need to burn fewer fossil fuels and set effective air quality standards.
In the US, the Clean Air Act of 1990 implemented pollution limits that helped cut SO2 emissions by 88% between 1990 and 2017. Additionally, air quality standards have also lowered nitrogen dioxide emissions by 50% in the same time period.
Landscapes that have been affected by acid rain can recover from its damage, however it takes time.
What is Acid Rain?
Acid Rain, as the name suggests, can be said as the precipitation of acid in the form of rain in the simplest manner. When atmospheric pollutants like oxides of nitrogen and sulphur react with rainwater and come down with the rain, then this results in Acid Rain.
Table of contents
- Definition Of Acid Rain
- Recommended Videos Of Acid Rain
- Causes Of Acid Rain
- Effect Of Acid Rain
- Real-Life Examples Acid Rain
- Prevention Of Acid Rain
- FAQs
Acid rain is made up of highly acidic water droplets due to air emissions, most specifically the disproportionate levels of sulphur and nitrogen emitted by vehicles and manufacturing processes. It is often called acid rain as this concept contains many types of acidic precipitation.
The acidic deposition takes place in two ways: wet and dry. Wet deposition is any form of precipitation which removes acids from the atmosphere and places them on the surface of the earth. In the absence of precipitation, dry deposition of polluting particles and gases sticks to the ground through dust and smoke.
Recommended Videos Of Acid Rain
Causes of Acid Rain
The causes of acid rain are Sulphur and Nitrogen particles which get mixed with the wet components of rain. Sulphur and Nitrogen particles which get mixed with water are found in two ways either man-made i.e as the emissions that are given out from industries or by natural causes like lightning strike in the atmosphere releasing nitrogen oxides and volcanic eruptions releasing sulphur oxide.
According to the Royal Society of Chemistry, which considers him the “father of acid rain,” the word acid rain was invented in 1852 by Scottish chemist Robert Angus Smith. Smith decided on the word while studying rainwater chemistry near industrial towns in England and Scotland.
The regular clean rain we experience, even though it is not clean i.e water and carbon dioxide react together to form weak carbonic acid which essentially by itself is not extremely harmful. The reaction occurring is :
H2O (l) + CO2 (g) ⇌ H2CO3 (aq)
The pH value of regular rainwater is around 5.7, giving it an acidic nature. The oxides of nitrogen and sulphur are blown away by the wind along with the dust particles. They settle on the earth’s surface after coming down in the form of precipitation. Acid rain is essentially a by-product of human activities which emit oxides of nitrogen and sulphur in the atmosphere. Example – the burning of fossil fuels, unethical waste emission disposal techniques.
Sulphur dioxide and nitrogen dioxide undergo oxidation, and then they react with water resulting in the formation of sulphuric acid and nitric acid, respectively. The following reaction will clarify the acid formation reaction:
2SO2 (g) + O2 (g) + 2H2O (l) → 2H2SO4 (aq)
4NO2 (g) + O2 (g) + 2H2O (l) → 4HNO3 (aq)
Effects of Acid Rain
- Acid rain is very harmful to agriculture, plants, and animals. It washes away all nutrients which are required for the growth and survival of plants. Acid rain affects agriculture by the way it alters the composition of the soil.
- It causes respiratory issues in animals and humans.
- When acid rain falls down and flows into the rivers and ponds it affects the aquatic ecosystem. It alters the chemical composition of the water, to a form which is actually harmful to the aquatic ecosystem to survive and causes water pollution.
- Acid rain also causes the corrosion of water pipes, which further results in leaching of heavy metals such as iron, lead and copper into drinking water.
- It damages the buildings and monuments made up of stones and metals.

Real-Life Examples
- Taj Mahal, one of the 7 wonders of the world, is largely affected by acid rain. The city of Agra has many industries which emit the oxides of sulphur and nitrogen in the atmosphere. People continue to use low-quality coal and firewood as a domestic fuel, adding to this problem. Acid rain has the following reaction with the marble (calcium carbonate):
CaCO3(s) + H2SO4(l) → CaSO4(s) + H2O(l) + CO2(g)
The formation of calcium sulphate results in the corrosion of this beautiful monument.
- Statue of Liberty which is made of copper has also been damaged by the cumulative action of acid rain and oxidation for over 30 years and is, therefore, becoming green.

Prevention of Acid Rain
- The only precaution that we can take against acid rain is having a check at the emission of oxides of nitrogen and sulphur.
- Acid rain is harmful to animals, plants and the monuments.
- Being responsible citizens, one should be aware of the harmful effects they cause and of the industries which give out nitrogen and sulphur compound wastes unethically.
Frequently Asked Questions – FAQs
Q1
What is acid rain and how is it caused?
Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
Q2
What are the effects of acid rain?
The ecological consequences of acid rain are seen most strongly in marine habitats, such as streams, lakes and marshes where fish and other wildlife can be toxic. Acidic rainwater can leach aluminium from soil clay particles as it flows through the soil and then floods into streams and lakes.
Q3
What will happen if we don’t stop acid rain?
Sulphur dioxide and nitrogen oxide are the principal chemicals for acid rain. It can also influence humans since the acid goes into fruits, vegetables and animals. In other words, we can get really sick if acid rain doesn’t stop, and we eat those things. In general, acid rain affects men, but not directly.
Q4
What is acid rain? What are its harmful effects?
It has been shown that acid rain has detrimental effects on trees, freshwaters and soils, destroys insects and aquatic life-forms, causes paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and sculptures, as well as impacts on human health.
Q5
What are three ways to reduce acid rain?
Alternative energy sources should be used, such as solar and wind power. Renewable sources of energy are helping to reduce acid rain, as they produce much fewer emissions. There are other electricity sources as well, such as nuclear power, hydropower, and geothermal energy. Among these, the most extensive use is among nuclear and hydropower.
Q6
How does acid rain affect plants?
Acid rain can affect the health of plants. Acid rain changes the pH of the land where the plant is growing, thereby affecting the overall growth of the plants. Moreover, it binds or dissolves essential soil minerals such as nitrogen and phosphorus and carries them away.
Q7
What is acid rain made of?
Acid rain comprises highly acidic water droplets due to air emissions, specifically the disproportionate levels of sulphur dioxide and nitrogen dioxide emitted by vehicles and manufacturing processes. Sulphur dioxide and nitrogen dioxide combine with water molecules to form sulphuric and nitric acid.
Q8
What is the primary source of acid rain?
The power plants primarily cause acid rain. It releases most of the sulphur dioxide and nitrogen dioxide while burning fossil fuels. Sulphur dioxide and nitrogen dioxide combine with water molecules to form sulphuric and nitric acid causing acid rain.
Q9
Can acid rain damage buildings?
Yes, acid rain harms buildings. It strips away the materials and corrodes the metals of the buildings. Example: Tarnishing of Taj Mahal.
Q10
Can acid rain burn your skin?
No, acid rain can not burn the skin.
Read more:
- Structure & Properties of Ozone
- Acids and Bases
- Global warming due to the greenhouse effect
Processes involved in acid deposition. Note that among the atmospheric pollutants shown, only sulfur dioxide (SO2) and nitrogen oxides (NOx) play a significant role in acid rain.
The term acid rain is commonly used to mean the deposition of acidic components in rain, snow, fog, dew, or dry particles. The more accurate term is acid precipitation. «Clean» or unpolluted rain is slightly acidic, because carbon dioxide and water in the air react together to form carbonic acid, a weak acid.[1] Rain acquires additional acidity through the reaction of air pollutants (primarily oxides of sulfur and nitrogen) with water in the air, to form strong acids (such as sulfuric acid and nitric acid). The main sources of these pollutants are emissions from vehicles, industrial plants, and power-generating plants.
Acid rain has been shown to have adverse effects on forests, freshwater, and soils, killing off insect and aquatic life forms. It also damages buildings and statues, and may adversely affect human health. These problems, which have increased with population and industrial growth, are being addressed by the use of pollution control equipment that reduces the emission of sulfur and nitrogen oxides.
History
Acid rain was first observed by Robert Angus Smith in Manchester, England. In 1852, he reported the relationship between acid rain and atmospheric pollution. It was, however, not until the late 1960s that scientists began widely observing and studying the phenomenon. Harold Harvey of Canada was among the first to research a «dead» lake. In the United States, public awareness of the problem was heightened in the 1990s, after the New York Times promulgated reports from the Hubbard Brook Experimental Forest in New Hampshire of the myriad deleterious environmental effects resulting from acid rain.
Since the Industrial Revolution, emissions of sulfur and nitrogen oxides to the atmosphere have increased. Industrial and energy-generating facilities that burn fossil fuels, primarily coal, are the principal sources of increased sulfur oxides. [2]
Emissions of chemicals leading to acidification
The most significant gas that leads to acidification of rainwater is sulfur dioxide (SO2). In addition, emissions of nitrogen oxides, which are oxidized to form nitric acid, are of increasing importance due to stricter controls on emissions of sulfur-containing compounds. It has been estimated that about 70 Tg(S) per year in the form of SO2 comes from fossil fuel combustion and industry, 2.8 Tg(S) per year comes from wildfires, and 7-8 Tg(S) per year comes from volcanoes.[3]
Human activity
The coal-fired Gavin power plant in Cheshire, Ohio.
Sulfur and nitrogen compounds are the principal causes of acid rain. Many of them are generated by human activity, such as electricity generation, factories, and motor vehicles. Coal power plants are among the most polluting. The gases can be carried hundreds of kilometers in the atmosphere before they are converted to acids and deposited.
Factories used to have short chimneys to release smoke, but because they polluted the air in their nearby localities, factories now have tall smokestacks. The problem with this «solution» is that those pollutants get carried far off, releasing gases into regional atmospheric circulation and contributing to the spread of acid rain. Often deposition occurs at considerable distances downwind of the emissions, with mountainous regions tending to receive the most (because of their higher rainfall). An example of this effect is the low pH of rain (compared to the local emissions) that falls in Scandinavia.
Chemistry in cloud droplets
When clouds are present, the loss rate of SO2 is faster than can be explained by gas phase chemistry alone. This is due to reactions in the liquid water droplets.
- Hydrolysis
Sulfur dioxide dissolves in water and then, like carbon dioxide, hydrolyzes in a series of equilibrium reactions:
- SO2 (g) + H2O ⇌ SO2·H2O
- SO2·H2O ⇌ H++HSO3—
- HSO3— ⇌ H++SO32-
- Oxidation
Many aqueous reactions oxidize sulfur from S(IV) to S(VI), leading to the formation of sulfuric acid. The most important oxidation reactions are with ozone, hydrogen peroxide, and oxygen. (Reactions with oxygen are catalyzed by iron and manganese in the cloud droplets).
Acid deposition
Wet deposition
Wet deposition of acids occurs when any form of precipitation (rain, snow, and so forth) removes acids from the atmosphere and delivers it to the Earth’s surface. This can result from the deposition of acids produced in the raindrops (see aqueous phase chemistry above) or by the precipitation removing the acids either in clouds or below clouds. Wet removal of both gases and aerosol are both of importance for wet deposition.
Dry deposition
Acid deposition also occurs via dry deposition in the absence of precipitation. This can be responsible for as much as 20-60 percent of total acid deposition. This occurs when particles and gases stick to the ground, plants, or other surfaces.
Adverse effects
Chart showing differing levels of acidity in water tolerated by a variety of species.
Surface waters and aquatic animals
Both the lower pH and higher aluminum concentrations in surface water that occur as a result of acid rain can cause damage to fish and other aquatic animals. At pH levels lower than 5, most fish eggs will not hatch, and lower pH levels can kill adult fish. As lakes become more acidic, biodiversity is reduced. There has been some debate on the extent to which man-made causes of lake acidity caused fish kills — for example Edward Krug determined that acid rain was an environmental nuisance, not a
catastrophe, and even that acid rain might not be the cause of lake acidity.[4]
Soils
Soil biology can be seriously damaged by acid rain. Some tropical microbes can quickly consume acids[5] but other microbes are unable to tolerate low pH levels and are killed. The enzymes of these microbes are denatured (changed in shape so they no longer function) by the acid.
Acid rain also removes minerals and nutrients from the soil that trees need to grow.[6]
Forests and other vegetation
Effect of acid rain on a forested area of the Jizera Mountains, Czech Republic.
Acid rain can slow the growth of forests, cause leaves and needles to turn brown and fall off and die. In extreme cases, trees or whole acres of forest can die. The death of trees is not usually a direct result of acid rain, but it often weakens trees and makes them more susceptible to other threats. Damage to soils (noted above) can also cause problems. High altitude forests are especially vulnerable as they are often surrounded by clouds and fog which are more acidic than rain.
Other plants can also be damaged by acid rain but the effect on food crops is minimized by the application of fertilizers to replace lost nutrients. In cultivated areas, limestone may also be added to increase the ability of the soil to keep the pH stable, but this tactic is largely unusable in the case of wilderness lands. Acid Rain depletes minerals from the soil and then it stunts the growth of the plant.
Human health
Some scientists have suggested direct links to human health, but none have been proven. However, fine particles, a large fraction of which are formed from the same gases as acid rain (sulfur dioxide and nitrogen dioxide), have been shown to cause problems with heart and lung function.[6]
Other adverse effects
Statues are damaged by acid rain.
Acid rain can also cause damage to certain building materials and historical monuments. This is because the sulfuric acid in the rain chemically reacts with the calcium compounds in the stones (limestone, sandstone, marble, and granite) to create gypsum, which then flakes off. This is also commonly seen on old gravestones where the acid rain can cause the inscription to become completely illegible. Acid rain also causes an increased rate of oxidation for iron, causing damage to metal structures and monuments.[6]
Prevention methods
Technological solutions
In the United States and various other countries, many coal-burning power plants use flue gas desulfurization (FGD) to remove sulfur-containing gases from their stack gases. An example of FGD is the wet scrubber, which is basically a reaction tower equipped with a fan that passes hot smoke stack gases through the tower. Lime or limestone in slurry form is also injected into the tower to mix with the stack gases and combine with the sulfur dioxide present. The calcium carbonate of the limestone produces pH-neutral calcium sulfate that is physically removed from the scrubber. In other words, the scrubber turns sulfur pollution into industrial sulfates.
In some areas, the sulfates are sold to chemical companies as gypsum when the purity of calcium sulfate is high. In others, they are placed in landfills. However, the effects of acid rain can last for generations, as the effects of pH level change can stimulate the continued leaching of undesirable chemicals into otherwise pristine water sources, killing off vulnerable insect and fish species and blocking efforts to restore native life.
International treaties
A number of international treaties have been signed regarding the long-range transport of atmospheric pollutants. One example is the Sulphur Emissions Reduction Protocol under the Convention on Long-Range Transboundary Air Pollution.
Emissions trading
A more recent regulatory scheme involves emissions trading. In this scheme, every current polluting facility is given an emissions license that becomes part of capital equipment. Operators can then install pollution control equipment, and sell parts of their emissions licenses. The intent here is to give operators economic incentives to install pollution controls.
See also
- Environmental engineering
- Nitrogen dioxide
- Sulfur dioxide
Notes
- ↑ Distilled water, which contains no carbon dioxide, has a neutral pH of 7. Liquids with a pH less than 7 are acidic, and those with a pH greater than 7 are alkaline (or basic).
- ↑ Acid rain. NASA Glossary. Retrieved June 13, 2018.
- ↑ H. Berresheim, P. H. Wine, and D. D. Davies, «Sulfur in the Atmosphere.» In Hanwant B. Singh (ed.), Composition, Chemistry and Climate of the Atmosphere (Wiley, 1995, ISBN 978-0471285144), 251-307.
- ↑ William Anderson, Acid Test: Edward Krug Flunks Political Science. The Reason Foundation, January 1992. Retrieved June 13, 2018.
- ↑ H. Rodhe, et al., «The Global Distribution of Acidifying Wet Deposition» Environmental Science & Technology 36(20) (2002): 4382-4388. Retrieved June 13, 2018.
- ↑ 6.0 6.1 6.2 Effects of Acid Rain EPA. Retrieved June 13, 2018.
References
ISBN links support NWE through referral fees
- McCormick, John. Acid Earth: The Global Threat of Acid Pollution. London, UK: Earthscan, 1989. ISBN 185383033X
- Morgan, Sally, and Jenny Vaughan. Acid Rain (Earth SOS). London, UK: Franklin Watts Ltd., 2007. ISBN 0749676728
- Parks, Peggy J Our Environment — Acid Rain (Our Environment). Farmington Hills, MI: KidHaven Press (Thomson Gale), 2005. ISBN 0737726288
- Singh, Hanwant B. (ed.). Composition Chemistry, and Climate of the Atmosphere. Wiley, 1995. ISBN 978-0471285144
External links
All links retrieved April 13, 2021.
- National Acid Precipitation Assessment Program Report — a 98-page report to Congress.
- Acid rain for schools.
- U.S. Environmental Protection Agency — Acid Rain
- U.S. Geological Survey — What is acid rain?
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article
in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Acid rain history
The history of this article since it was imported to New World Encyclopedia:
- History of «Acid rain»
Note: Some restrictions may apply to use of individual images which are separately licensed.