Товары на складе

EX-CELL® Advanced CHO Fed-batch Medium
Код:
24366C-10L
Марка:
Sigma-Aldrich
В наличии:
6 шт
Цена с НДС:
3420€
Заказать
Описание
Описание
Технические данные
Доставка
Описание
Технические данные
Доставка
Другие товары в наличии
L030015. Пробирки центрифужные типа фалькон 15 мл
Plastic centrifuge tubes Transparent high quality PP material for laboratory hospitals. Код: …
Подробнее
L030050. Пробирки центрефужные типа фалькон 50 мл
Plastic centrifuge tubes Transparent high quality PP material for laboratory hospitals. Код: …
Подробнее
8187370100. Хлортриметилсилан CAS 75-77-4
Chlorotrimethylsilane. Код: 8187370100. Марка: Sigma …
Подробнее
- 1
- 2
- 3
- 4
- 5
- 6
- 7
L030015. Пробирки центрифужные типа фалькон 15 мл
Plastic centrifuge tubes Transparent high quality PP material for laboratory …
Подробнее
L030050. Пробирки центрефужные типа фалькон 50 мл
Plastic centrifuge tubes Transparent high quality PP material for laboratory …
Подробнее
8187370100. Хлортриметилсилан CAS 75-77-4
Chlorotrimethylsilane. Код: 8187370100. Марка: Sigma …
Подробнее
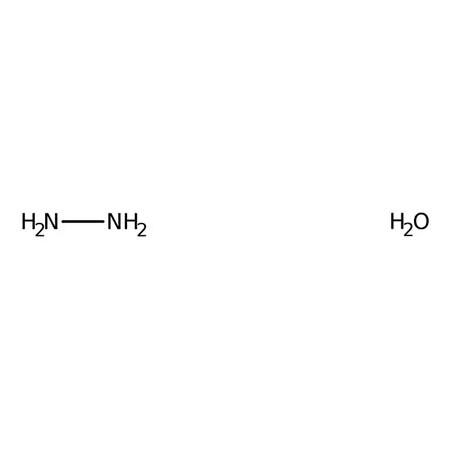
H/0550/PB17. Гидразингидрат, 99+%, особо чистый, SLR
Hydrazine hydrate, 99+%, extra pure, 500ml. Код: H/0550/PB17. Марка: Thermo …
Подробнее
430052. Пробирка типа фалькон 15 мл
Corning™ Polypropylene Centrifuge Tubes, Sterile. Код: 430052. Марка: Corning …
Подробнее
430897. Пробирка типа фалькон 50 мл
Corning™ Polypropylene Centrifuge Tubes, Sterile. Код: 430897. Марка: Corning …
Подробнее
87050. Биореакторы TubeSpin 50 мл (минитьюбы)
MIT (минитьюбы). TPP™ TubeSpin™ Bioreactors. Код: 87050. Марка: TPP …
Подробнее
24366C-10L. Среда Advanced CHO подпитка для клеток 10 л
EX-CELL Advanced CHO Fed-batch Medium. Код: 24366C-10L. Марка: Sigma-Aldrich. …
Подробнее
24366C-100L. Среда Advanced CHO подпитка для клеток 100 л
EX-CELL Advanced CHO Fed-batch Medium. Код: 24366C-100L. Марка: Sigma-Aldrich. …
Подробнее
Поставка от производителя или с нашего склада
Часть позиций Вы сможете найти в наличии на нашем складе в Санкт-Петербурге или заказать поставку от производителя
Товары в наличии
Отправить запрос:
Ваше имя:
Телефон:
Удобный способ связи:
Детали запроса:
Контакты
Наши менеджеры всегда на связи с 9:00 до 18:00 Пн-Пт.
Телефон:
8(812) 242-93-60
E-mail:
zakaz@a-farma.ru
Адрес:
194100, г.Санкт-Петербург, ул.Новолитовская, д.15 лит.А
Содержание
- SAFC Launches New EX-CELL® Advanced™ Products for Scalable, Efficient Biopharmaceutical Manufacturing
- Share this article
- Semi-Automated Glycoproteomic Data Analysis of LC-MS Data Using GlycopeptideGraphMS in Process Development of Monoclonal Antibody Biologics
- Introduction
- Materials and Methods
- Chemicals, Materials and Reagents
- Production and Isolation of In-House Produced Trastuzumab Biosimilar
- Proteolytic Digestion of Antibodies
- LC-MS of Tryptic Digested Glycopeptides
- BioAccord LC-MS System Data Preprocessing
- GraphMS Analysis
- N-glycan Release and Labeling
- LC-MS of Released N-glycans
- N-glycans Assignment
- Statistical Analyses
- GraphMS Data Analysis of Trastuzumab Biosimilar Fermentation Generates Longitudinal Characterisation of Bioprocess and Biologic Glycosylation
SAFC Launches New EX-CELL® Advanced™ Products for Scalable, Efficient Biopharmaceutical Manufacturing
News provided by
Apr 07, 2015, 07:00 ET
Share this article
ST. LOUIS , April 7, 2015 /PRNewswire/ — Sigma-Aldrich Corporation (NASDAQ: SIAL) today announced that SAFC ® Commercial (www.sigma-aldrich.com/safc), the Company’s business unit providing products and services for use in regulated pharmaceutical and biopharmaceutical applications, has launched the EX-CELL ® Advanced™ product line. Designed to address the needs of an evolving industry where speed to market is paramount, the new product line provides for increased performance, streamlines regulatory compliance, and offers the supply chain security needed in today’s biopharmaceutical environment.
The first EX-CELL Advanced product is the high-performing batch media system developed for a range of widely used industrial CHO cell lines, including SAFC’s proprietary CHOZN ® cell line. During rigorous testing, the EX-CELL Advanced CHO Fed-batch System outperformed other commercially available equivalents by displaying significantly higher titers. Robust scale up and effortless adaptation offers those in biopharmaceutical development the opportunity to get their process up and running quickly and efficiently. Protein quality, another vital consideration in the development process, remains uncompromised.
«At SAFC we recognize our customers’ needs are constantly changing», said Deb Stutz , Director of Biopharm Marketing. «Our team has utilized unique expertise in raw material science, understanding of process knowledge and decades of experience in cGMP manufacturing in development of the new EX-CELL Advanced line. We are delighted to launch this product in response to market needs for new levels of speed and performance, while continuing to provide the confidence of a secure and transparent supply chain.»
Источник
Semi-Automated Glycoproteomic Data Analysis of LC-MS Data Using GlycopeptideGraphMS in Process Development of Monoclonal Antibody Biologics
The glycosylation of antibody-based proteins is vital in translating the right therapeutic outcomes of the patient. Despite this, significant infrastructure is required to analyse biologic glycosylation in various unit operations from biologic development, process development to QA/QC in bio-manufacturing. Simplified mass spectrometers offer ease of operation as well as the portability of method development across various operations. Furthermore, data analysis would need to have a degree of automation to relay information back to the manufacturing line. We set out to investigate the applicability of using a semiautomated data analysis workflow to investigate glycosylation in different biologic development test cases. The workflow involves data acquisition using a BioAccord LC-MS system with a data-analytical tool called GlycopeptideGraphMS along with Progenesis QI to semi-automate glycoproteomic characterisation and quantitation with a LC-MS1 dataset of a glycopeptides and peptides. Data analysis which involved identifying glycopeptides and their quantitative glycosylation was performed in 30 min with minimal user intervention. To demonstrate the effectiveness of the antibody and biologic glycopeptide assignment in various scenarios akin to biologic development activities, we demonstrate the effectiveness in the filtering of IgG1 and IgG2 subclasses from human serum IgG as well as innovator drugs trastuzumab and adalimumab and glycoforms by virtue of their glycosylation pattern. We demonstrate a high correlation between conventional released glycan analysis with fluorescent tagging and glycopeptide assignment derived from GraphMS. GraphMS workflow was then used to monitor the glycoform of our in-house trastuzumab biosimilar produced in fed-batch cultures. The demonstrated utility of GraphMS to semi-automate quantitation and qualitative identification of glycopeptides proves to be an easy data analysis method that can complement emerging multi-attribute monitoring (MAM) analytical toolsets in bioprocess environments.
Introduction
Biologic glycosylation impacts efficacy, function, and clinical outcomes of the molecule. For example, with antibody drugs, the fucosylation or galactosylation heavily impacts the antibody cytotoxicity or complement-dependent cytotoxicity respectively (Shields et al., 2002; Hodoniczky et al., 2005). There is an increase rate of new approved monoclonal antibody (mAb) therapeutic products from different IgG subclasses (Kaplon and Reichert, 2021). Each IgG subclass can exhibit different degrees of glycosylation (Lippold et al., 2020). Ensuring the correct glycosylation of the product is therefore a major challenge within bioprocessing operations. While glycosylation can be altered significantly via cell-line engineering (Goh et al., 2014), there is still much to learn about how glycosylation can be manipulated using different cultured conditions during cell fermentation (Walsh et al., 2020). For example, there have been studies that demonstrated that dissolved oxygen tension, pH, NH4 + , temperature and nutrient supplementation during upstream culturing can affect the glycan profile of mAb (Zhang et al., 2016). Additionally, Schiestl et al. (2011) analysed different lots of Rituxan® and found that differences in the abundance of afucosylated G0 gylcan in different batches had an impact of antibody-dependent cellular cytotoxicity (ADCC); the difference in fucosylation was attributed to batch-to-batch variability in the manufacturing process (Schiestl et al., 2011). These challenges during lead development, or clone selection activities, highlight the importance to relay information such as degree of glycosylation in an expedient manner to ensure an efficient decision-making process.
To that end, the availability of a robust and sensitive glyco-analytical platform such as that centered around liquid chromatography-mass spectrometry (LC-MS) are being implemented to facilitate detailed understanding of glycosylation and bioprocessing operation (Tsai and Chen, 2017). With regards to mass spectrometry (MS), intact glycans/glycopeptides can be conventionally identified via their intact mass in MS and the nature of their fragmentation in MS/MS mode. Measuring a glycan/glycopeptides intact mass (MS) can often be enough to elucidate the composition of the molecule of interest. Observing the fragmentation of intact molecules (MS/MS) is often straightforward to elucidating the linear sequence of molecules such as proteins and oligonucleotides. With respect to fragmentation of glycans, the resultant isobaric masses, only confirm the composition of the glycan (in positive mode) and do little in the way of offering information such as their branching, topology and isomerism without significant effort with advanced glycomic techniques (Zhou et al., 2017). Recent additions to configurations of mass spectrometers mean that other layers of information with which to help characterise the glycoconjugates. Electron transfer dissociation (ETD), for example, has been used effectively to identify the site of the glycan on the glycopeptide (Riley and Coon, 2018). Additionally, ion mobility has recently been used to characterise glycan arm isomers (Pallister et al., 2020). As one can appreciate, significant expertise and effort as well as advanced mass specrometers with higher specifications are often needed to resolve more information about a glyco-conjugates’ structure.
The complexity of LC-MS systems for glycopeptide analysis has necessitated the use of simplified mass spectrometers and analytical workflows capable of performing analyses at intact sub-unit, peptide and released glycan level with optimised compliance-ready workflows (Rogers et al., 2018). These workflows are known as multi-attribute monitoring (MAM) and they can be performed with mass spectrometers and have been used to identify biotherapeutic attributes such as deamidation, oxidation, pyroglutamate formation, lysine clipping, aspartate isomerization and glycosylation in one analysis (Song et al., 2021). In most instances, the accurate mass of the peptides and PTMs are compared and validated by cross-referencing these measurements to a curated database. MAM is being increasingly adopted within the biopharmaceutical industry because in principle the analytical workflows which include sample preparation and data processing techniques and instrumentation are robust enough to be harmonized across the biologic development value chain and across different manufacturing sites (Song et al., 2021). This harmonization decreases the cost of assay development; however, there is the potential challenge that these techniques can miss unexpected PTMs such as glycosylation which may not be described in the analytical databases. Therefore, a database –independent approach would be ideal to identify and profile this unexpected glycosylation.
LC-MS technologies generate large volumes of data and data analysis approaches are the largest bottleneck to relaying glycosylation information in a timely manner. Several approaches to decipher or process information from these configurations are quite involved and there exist numerous efforts to facilitate this. Extensive reviews of the software and informatics resources to help with glycan analysis can be found here (Hu et al., 2016; Walsh et al., 2016; Campbell, 2017; Abrahams et al., 2020). In principle, data analysis for MS based glycoproteomic can be summarized into a few strategies; one of which is the accurate mass measurement of the glycopeptide using MS data and deduction of the glycan composition based on the MS/MS fragments. These solutions often require matching measured masses and their fragments to a curated or simulated glycan and peptide database for confirmation. These approaches therefore make it difficult to identify unexpected glycan compositions that may not be described in databases. Furthermore, they often depend quite heavily upon human intervention and interpretation of the results. Recently, informatic approaches such as the application of GlycopeptideGraphMS (GraphMS) have exploited patterns in glycopeptide detection in a LC-MS to analyse glycopeptides (Choo et al., 2019). GraphMS is a graph theoretical algorithm that groups LC-MS features into grouped networks that pertain to N-glycopeptides sharing the same peptide backbone. Doing so offers higher confidence in characterisation and validation of glycopeptides in any complex sample. It has previously been exploited to comprehensively characterise AXL protein, a glycoprotein with higher complex glycosylation heterogeneity compared to antibody molecules, and IgG (Choo et al., 2019; Lippold et al., 2020).
In this study, we explore the utility of GraphMS on data generated from a BioAccord LC-MS system—a system that is simple to operate for LC-MS expert and non-expert alike without compromising the quality, resolution and accuracy of data—in several use cases that represent the value chain of a bioprocessing operation that produces the IgG biosimilar trastuzumab (anti-HER-2). We intend on using a workflow that involves collecting data from the instrument, deconvolution through Progenesis QI and subsequent glycopeptide mapping with GraphMS to characterise the glycopeptides and their compositions qualitatively and quantitatively. This approach is robust, is database-independent and requires very little user intervention other than to confirm the mass of reference nodes. We apply this approach to different biologic development scenarios and compare this technique to conventional fluorescently labeled released glycan analysis protocols.
Materials and Methods
Chemicals, Materials and Reagents
Serum IgG was purchased from Sigma-Aldrich (United States). Trastuzumab (Herceptin), and adalimumab (Humira) were purchased from Roche (Switzerland), and Abbott (United States), respectively. Unless otherwise stated, all chemicals and reagents were purchased from Sigma-Aldrich (United States).
Production and Isolation of In-House Produced Trastuzumab Biosimilar
Trastuzumab biosimilar was produced in CHO-K1 cells cultured in 14-days fed-batch cultures using Ambr250 bioreactors. Each fed-batch culture was started by inoculation of cells into 200 ml of EX-CELL Advanced CHO Fed-batch medium (SAFC media, Sigma Aldrich, United States) or HyClone ActiPro (GE media, GE Healthcare, United States) cell culture media supplemented with 6 mM of glutamine but without MTX. The inoculated viable cell density was 3 × 10 5 cells/ml. The cultures were maintained at 37°C. Dissolved oxygen was maintained at 50%, while pH was maintained at 7.1. The cultures were mixed using dual pitch blade impellers stirring at 300 rpm. For cultures in EX-CELL Advanced CHO Fed-batch medium, 10% of EX-CELL Advanced CHO Feed 1 (with glucose) were fed into culture at day 3, 5, 7, 9 and 14. For cultures in HyClone ActiPro media, 6% Cell boost 7a and 0.6% Cell boost 7b were fed into culture at day 3, 5, 7, 9 and 14. Glucose concentration in the media was analysed on days 3, 5, 7, 9 and 14 via the Nova BioProfile 100 plus bioanalyser (Nova Biomedical, United States). When the concentration of glucose dropped to below 2 g/L, a specified volume of 45% glucose stock was added into fed-batch cultures in order to achieve final glucose concentration at 6 g/L. Media samples were taken at days 3, 5, 7, 9 and 14 for glycosylation analysis. Briefly, the media samples were filtered using a 0.45 μm membrane filter (MerckMillipore, United States) and concentrated using Amicon Ultracel-10k centrifugal filter units (MerckMillipore, United States) before trastuzumab biosimilar was purified using Protein A HP SpinTrap column (GE Healthcare, United States). Purified trastuzumab biosimilar was buffer-exchanged into water using Amicon Ultracel-10k centrifugal filter units to eliminate any salts and nucleophiles prior to drying them using a CentriVap centrifugal vacuum concentrator (Labconco, United States).
Proteolytic Digestion of Antibodies
Proteolytic digestion of antibody was performed using the modified single-pot, solid-phase-enhanced sample preparation (SP3) technology as described previously (Hughes et al., 2019). Briefly, 20 μg of dried antibody was denatured in 15 μL of 8 M Urea (dissolved in 50 mM ammonium bicarbonate), followed by 30 min incubation at 60°C with 10 μL of dithiothreitol (DTT, dissolved in 50 mM ammonium bicarbonate). 10 μL of 150 mM iodoacetamide (dissolved in 50 mM ammonium bicarbonate) was then added to the sample and incubated at room temperature in the dark for 30 min. Fifteen microlitre (15 μl) of DTT was added to quench the alkylation. Two hundred micrograms (200 μg) of Sera-Mag SpeedBead carboxylate-modified magnetic particles (GE Healthcare, United States) were added to the sample, followed by pure acetonitrile (ACN) to give a final concentration of 70% ACN. The sample was incubated at room temperature for 10 min to allow protein binding. Magnetic particles were immobilised on the side of the tube by placing it on a magnetic rack to remove supernatant, followed by three washes with 80% ACN. Mass Spectrometry grade trypsin/Lys-C mix (dissolved in 50 mM ammonium bicarbonate, Promega Corporation, United States) in a 1:20 trypsin/LysC:protein ratio was added to the sample and incubated at 37°C for 16 h. The supernatant was collected, and digested glycopeptides were dried and reconstituted in LCMS grade water for LC-MS analysis.
LC-MS of Tryptic Digested Glycopeptides
Five micrograms (5 μg) of tryptic digested antibody were analysed on a BioAccord LC-MS system (Waters Corporation, United States). The system configuration includes an ACQUITY UPLC I-Class PLUS with an ACQUITY RDa detector (a compact time-of-flight mass detector) controlled by the compliance-ready UNIFI Scientific Information System software platform (Waters Corporation, United States). Samples were separated using an ACQUITY UPLC Peptide BEH C18 column (130 Å, 1.7 μm, 2.1 mm × 100 mm, Waters Corporation, United States) at 65°C and 250 μL/min, with a 60 min gradient from 1 to 40% of 0.1% formic acid in acetonitrile (mobile phase B). 0.1% formic acid in LCMS water was used as mobile phase A. The RDa mass detector was operated in positive electrospray ionisation full scan with fragmentation acquisition mode (MSe) at 2 Hz acquisition rate with a mass range of 400–2,000 m/z. The capillary voltage was set at 1.2 kV, cone voltage at 30 V, and the desolvation temperature was kept at 350°C. Leucine Enkephalin (Waters Corporation, United States) was used as the LockSpray compound for real-time mass correction.
BioAccord LC-MS System Data Preprocessing
Data obtained from the BioAccord LC-MS system were exported as .uep file using the UNIFI Scientific Information System (Waters Corporation, United States). LCMS features from the data were extracted, deconvoluted, and exported as a .csv file listing each feature with its retention time, neutral mass, and intensity using Progenesis QI software (Waters Corporation, United States). As the smallest glycopeptide (EEQY N STYR or EEQF N STFR with HexNAc) has a neutral mass of 1359.594, data points with a neutral mass lower than that were removed from the list.
GraphMS Analysis
Preprocessed .csv file was analysed using GraphMS software for glycopeptide identification as described previously (Choo et al., 2019). GraphMS plotted LC-MS features (neutral mass vs. retention time) into the nodes of a graph network. GraphMS’s algorithm then detected nodes that have a neutral mass difference that matched a predefined list of masses of Hex, HexNAc, Fuc, HexHexNAc, and NeuAc. Pairs of nodes were selected within a defined retention time window (30 s for Hex, HexNAc, Fuc, and NeuAc; 50 s for HexHexNAc; 500 s for NeuAc). These identified nodes with proximate retention time were clustered into subgraphs and connected by graph theoretic edges. Glycan composition and peptide of the reference node of each subgraph was manually assigned if it matches the modified “N-glycan 309 mammalian no sodium” database. The database was modified by adding the neutral mass of peptides to the neutral mass of glycans to form a list of glycopeptide neutral mass [for example, the neutral mass of EEQY N [+N4H3]STYR (2486.981)is the sum of the mass of EEQY N STYR (1188.505) and N4H3 (1298.476)], GraphMS then automatically identified the remaining nodes that are connected to the reference node by tracing the path of known monosaccharide mass additions or subtractions. Intensity of glycan and glycosylation attributes are presented as relative abundance in percentage [intensity of a glycan (or sum of glycan with attribute of interest)/sum of intensity x 100%]. The output of GraphMS consists of an excel spreadsheet that has a list of assigned glycans composition and intensities which corresponds to abundance.
N-glycan Release and Labeling
N-glycan of antibodies were released and labeled using GlycoWorks RapiFluor-MS (RFMS) N-glycan kit (Waters Corporation, United States) according to the manufacturer’s protocol. Briefly, 15 μg of dried antibody was reconstituted in 22.8 μL of LCMS grade water and 6 μL of 5% RapiGest solution (Waters Corporation, United States). The solution was incubated at 95°C for 5 min to denature the antibody. N-glycans were released enzymatically by adding 1.2 μL (600 U) of Rapid PNGase F (Waters Corporation, United States) followed by 10 min incubation at 55°C. Released N-glycans were labeled with 12 μL of the RapiFluor-MS Reagent Solution (fluorescence label) at room temperature for 5 min. The solution was diluted in 358 μL of ACN, followed by isolation using a GlycoWorks HILIC μElution Plate (Waters Corporation, United States). Isolated released N-glycans were dried and reconstituted in 9 μL of LCMS grade water, 10 μL of DMF, and 21 μL of ACN sequentially for LC-MS analysis.
LC-MS of Released N-glycans
Ten microlitres (10 μL) of reconstituted released N-glycans were injected into an ACQUITY H-Class UPLC (Waters Corporation, United States) coupled to a Xevo G2S QTof mass spectrometer (Waters Corporation, United States). Samples were separated using an ACQUITY UPLC Glycan BEH amide column (130 Å, 1.7 μm, 2.1 mm × 150 mm, Waters Corporation, United States) at 60°C and 400 μL/min, with a 40 min gradient from 25 to 49% of 50 mM Ammonium Formate (mobile phase A). 100% ACN was used as mobile phase B. RFMS-labelled glycans were excited at 265 nm and measured at 425 nm with an ACQUITY UPLC FLR detector (Waters Corporation, United States). The MS1 profile scans of m/z 400–2,000 were acquired using the Xevo G2S-QTof in positive mode with an acquisition rate of 1 Hz. The electrospray ionisation capillary voltage was set at 2.75 kV, cone voltage at 15 V, desolvation gas flow at 800 L/h, ion source temperature and desolvation temperature were kept at 120°C and 300°C, respectively. Glu1-fibrinopeptide B (Waters Corporation, United States) was used as the LockSpray compound for real-time mass correction. RapiFluor-MS Dextran Calibration ladder (Waters Corporation, United States) was also injected into LC-MS to calibrate the retention time of sample peaks. The retention times were normalised using the dextran calibration curve to Glucose Units (GU).
N-glycans Assignment
Released N-glycans data were analysed using the UNIFI Scientific Information System. Fluorescence peaks were integrated manually using the UNIFI Scientific Information System and relative quantitation of peaks was obtained by area-under-curve measurements followed by normalisation to the total area. Glycan assignment was done by matching Glycan Unit (GU) and m/z of each peak to the relevant UNIFI library.
Statistical Analyses
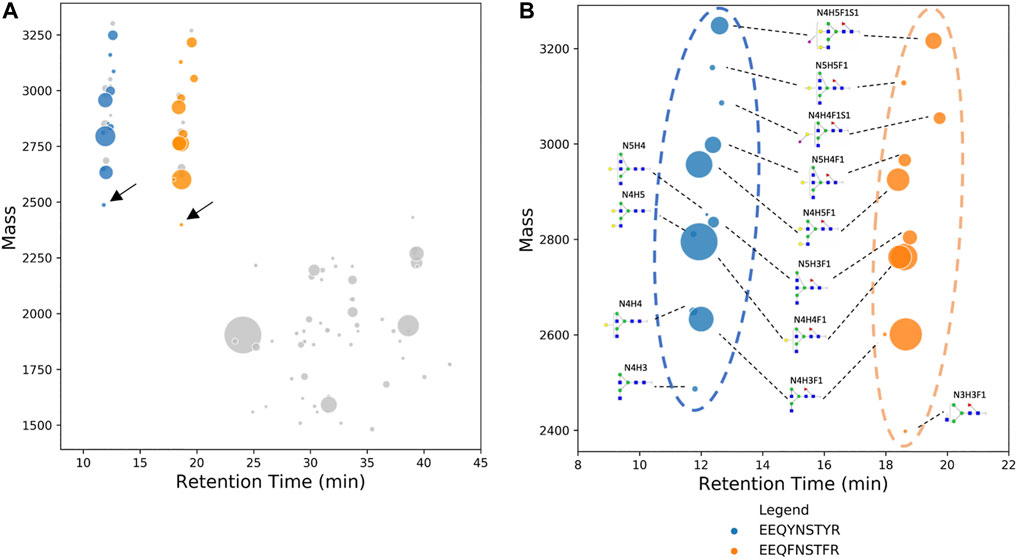
Data are presented as mean ± standard error of the mean. Statistical analyses were performed by Student’s t-test, one-way ANOVA, or two-way ANOVA with Bonferroni’s post hoc test using GraphPad Prism 8 (GraphPAD Software Inc., United States). Multiple comparisons were performed between IgG subclasses, antibody groups, or culture media in ANOVA tests. Data obtained from Xevo G2-S QTof-UNIFI workflow and BioAccord-GraphMS workflow were pooled (three per antibody group) and analysed using linear regression analysis. The criterion for significance was p N [+N4H3]STYR (IgG1, neutral mass 2486.981) glycopeptide and EEQF N [+N3H3F1]STFR (IgG2, neutral mass 2429.959) glycopeptide of human serum IgG. Glycopeptides EEQY N STYR (IgG1) and EEQF N STFR (IgG2) can be separated well as demonstrated in Figure 2B and they eluted at approximately 12 and 18 min, respectively; this is due to the difference in their hydrophobicity of the peptide backbone (Meek, 1980). Two distinct clusters can be identified and differentiated visually from dataset of glycopeptides and peptides.
FIGURE 2. (A) Deconvoluted mass of human serum IgG glycopeptides are plotted on a bubble chart against their respective retention time. Nodes with the lowest neutral mass in each subgraph, also known as reference nodes, are compared against the database of IgG glycopeptide masses. Two reference nodes (labeled with arrows), among other non-glycopeptide nodes (in gray), matched IgG glycopeptide mass and their composition was manually assigned. (B) Bubble chart of the deconvoluted mass of glycoforms of EEQY N STYR (human serum IgG1) and EEQY N STYR (human serum IgG2) against their respective retention time. Identification of glycan composition was performed using GraphMS. The plot shows a clear distinction between IgG1 and IgG2. In some cases, glycopeptides are plotted separately as two nodes due to the slight difference in retention time (N4H3F1 and N4H4F1 of EEQY N STYR).
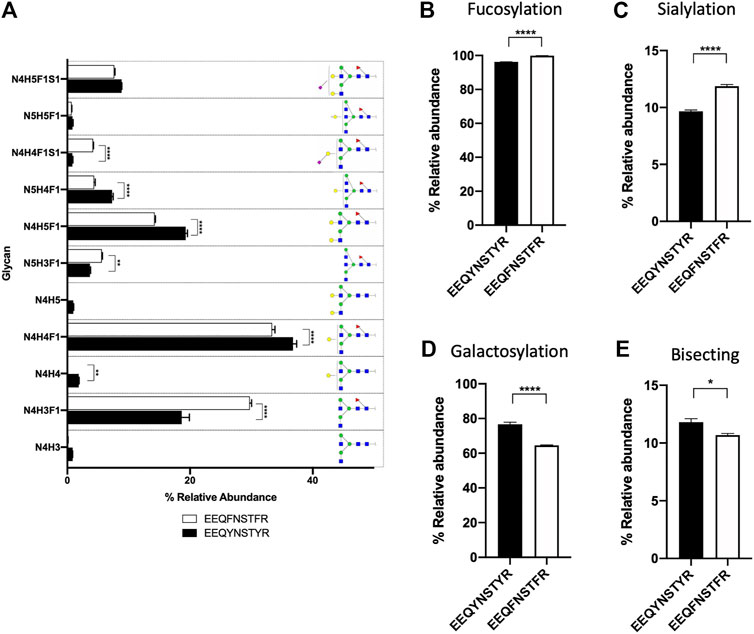
Remaining nodes in each subgraph were identified automatically and without referencing any glycan or peptide database, as discussed in the previous section, and their glycan compositions are annotated in Figure 2B. In addition to composition assignment, GraphMS also plots intensity on the third dimension- bubble size, which corresponds to the abundance of the molecules. Thus, the visualisation shown in Figure 2B is a new way to present and quickly compare two IgG subclasses qualitatively and quantitatively. The relative abundance of each glycan on EEQY N STYR (IgG1) and EEQF N STFR (IgG2) peptides is summarised in Figure 3A (only those with relative abundance higher than 0.5% are plotted).
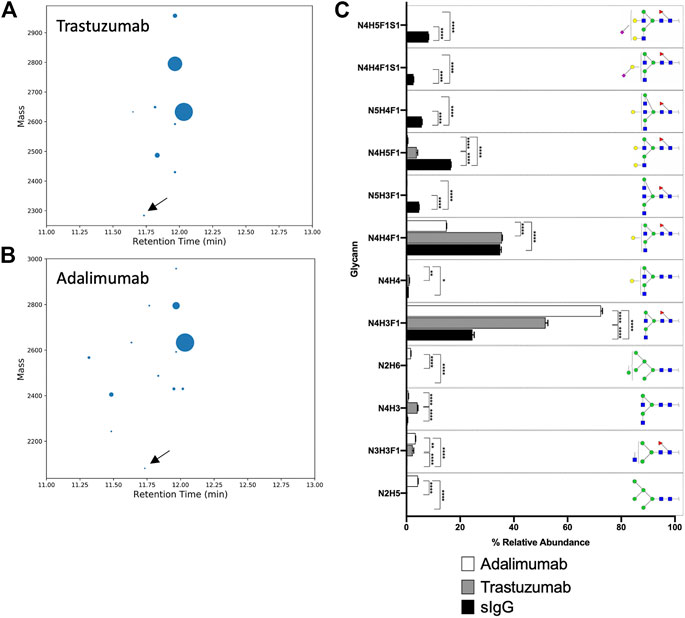
FIGURE 3. (A) Bar chart summarises the relative abundance (in percentage) of glycans of EEQY N STYR and EEQY N STYR (only those with relative abundance higher than 0.5% are plotted). Relative abundance of (B) fucosylation, (C) sialylation, (D) galactosylation, and (E) bisecting structure of serum IgG. (A), Two-way ANOVA followed by Bonferroni’s post hoc test, multiple comparisons were performed between EEQY N STYR and EEQY N STYR, n = 4; (B–E), Student’s t-test; n = 4, *p N STYR (IgG1) and EEQF N STFR (IgG2) peptides was found to be significantly different. For example, relative abundance of two most abundant glycans- N4H4F1 and N4H5F1, on EEQY N STYR (IgG1) were significantly higher than EEQF N STFR (IgG2) (Figure 3A, 36.782 ± 0.594% versus 33.368 ± 0.471%, p N [+N3H3]STYR and EEQY N [+N2H3]STYR, respectively, and their compositions were manually assigned. GraphMS then automatically assigned the composition of the remaining nodes. The relative abundance of glycoforms of trastuzumab and adalimumab, together with those of human serum IgG, is summarised in Figure 4C (only those with relative abundance higher than 0.5% are plotted).
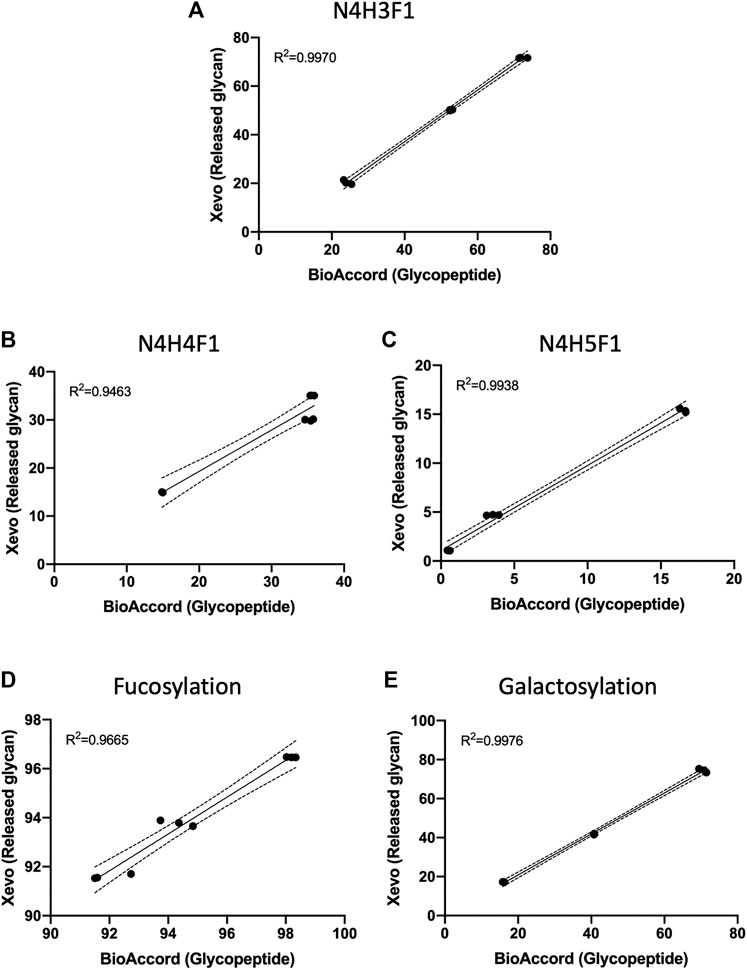
FIGURE 4. Bubble chart of the deconvoluted mass of glycoforms of EEQY N STYR of (A) trastuzumab, and (B) adalimumab, against their respective retention time. Identification of glycan composition was performed using GraphMS. Reference nodes of trastuzumab and adalimumab are labeled with black arrows. (C) Bar chart summarises the relative abundance (in percentage) of glycans of serum IgG, trastuzumab, and adalimumab (only those with relative abundance higher than 0.5% are plotted). (C), Two-way ANOVA followed by Borreroni’s post hoc test, multiple comparisons were performed between serum IgG, transtuzumab, and adalimumab, n = 4; *p 2 = 0.9970, 0.9463, and 0.9938, respectively). Similarly, glycosylation attributes like fucosylation and galactosylation were also found to be highly correlated (Figures 6D,E, R 2 = 0.9665, and 0.9976, respectively).
FIGURE 6. Data of the three most abundant glycans, (A) N4H3F1, (B) N4H4F1, and (C) N4H5F1, and glycosylation attributes, (D) fucosylation and (E) galactosylation, acquired and analysed using Xevo G2-S QTof workflow were highly correlated to BioAccord LC-MS system workflow. (Linear regression analysis, three replicates per antibody group, n = 9).
This demonstrates that the relative abundances of ion-intensity of a glycopeptide have high correlation to the stoichiometric abundance of glycans in both workflows. This comparison also supports the use of glycopeptide ion-intensity as a direct measure of glycosylation in multi-attribute monitoring (MAM) workflows that are emerging within the bio-analytical industry. The GraphMS data analysis workflow does semi-automatically generate this from collating the extracted ion intensities of the charge states of the candidate glycopeptide mass and combines this during the deconvolution stage (via Progenesis QI).
GraphMS Data Analysis of Trastuzumab Biosimilar Fermentation Generates Longitudinal Characterisation of Bioprocess and Biologic Glycosylation
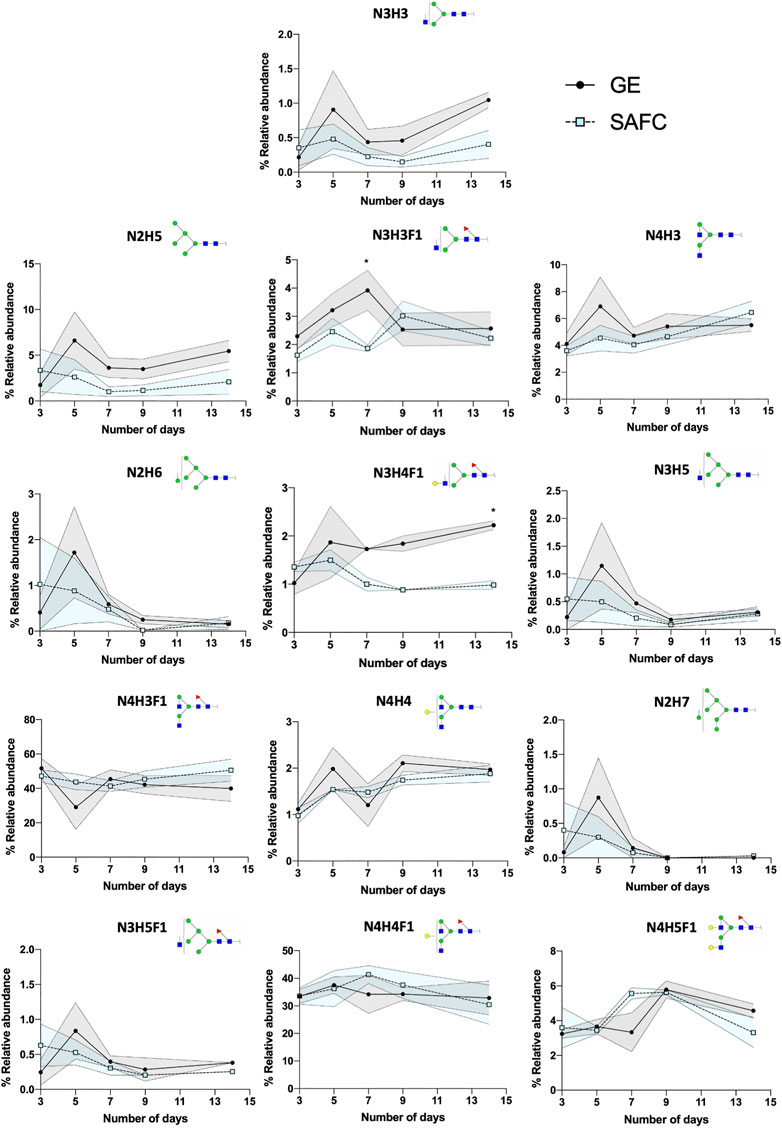
We set to demonstrate the utility of our described workflow in investigating the effect of different culturing conditions on the glycosylation of an in-house cultivated trastuzumab (anti-HER-2 antibody) biosimilar. This data is crucial to supplement studies and decision-making process in the cost, time efficiencies of production and importantly the safety profiles of the biologic. Hence, an efficient workflow like BioAccord-GraphMS allows the identification and quantitation would be useful when dealing with a multitude of data every other day of the bioprocessing operation. Trastuzumab antibodies were produced in either GE media or SAFC media in a fed-batch bioreactor and were harvested every 2nd day from days 3 to 14. Tryptic digested anti-HER-2 antibodies were injected into BioAccord LC-MS system and the LC-MS data were analysed using GraphMS.
The canonical antibody glycopeptide EEQY N STYR along with its resident glycans were identified, quantified, and monitored using GraphMS workflow. There were little differences in the abundance of the glycopeptides observed between the antibodies in different cultured media on the final day (day 14) of cultivation except for glycopeptide N3H3, N2H5, and N3H4F1 (Figure 7, 1.045 ± 0.112 versus 0.4028 ± 0.204, 5.454 ± 1.167 versus 2.093 ± 1.356, and 2.222 ± 0.091 versus 0.981 ± 0.088, respectively). Here, the glycosylation was increased in glycopeptides harvested in GE media compared to SAFC. Amongst the individual glycopeptides, the separation between GE and SAFC were most distinguished between N3H3F1 and N4H5F1 at around day 7 (Figure 7). These glycopeptides returned to comparable levels of glycopeptide abundance between the two media.
FIGURE 7. The relative abundance of in-house produced trastuzumab biosimilar glycoforms cultured in GE or SAFC media and on different length of duration. (Two-way ANOVA followed by Bonferroni’s post hoc test, multiple comparisons were performed between GE and SAFC media, n = 3; *p Keywords: GraphMS, BioAccord, IgG, trastuzumab, adalimumab, glycoproteomic, process development, biologics
Citation: Pang KT, Tay SJ, Wan C, Walsh I, Choo MSF, Yang YS, Choo A, Ho YS and Nguyen-Khuong T (2021) Semi-Automated Glycoproteomic Data Analysis of LC-MS Data Using GlycopeptideGraphMS in Process Development of Monoclonal Antibody Biologics. Front. Chem. 9:661406. doi: 10.3389/fchem.2021.661406
Received: 30 January 2021; Accepted: 30 April 2021;
Published: 18 May 2021.
Tara Louise Pukala, University of Adelaide, Australia
David Falck, Leiden University Medical Center, Netherlands
Quezia B. Cass, Federal University of São Carlos, Brazil
Copyright © 2021 Pang, Tay, Wan, Walsh, Choo, Yang, Choo, Ho and Nguyen-Khuong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
This article is part of the Research Topic
Technical and Methodological Advances in Proteomics
Источник
Get more Bioprocess insight with our FREE newsletter
sign me up
Datasheet
| April 26, 2021
EX-CELL® Advanced CHO Fed-Batch Medium is chemically defined, animal component free, and contains no hydrolysates or components of unknown composition. The formulations was developed using mulitvariate analysis of 10,000+ data points that included performance, physical, regulatory and safety design specifications.
This medium supports high growth and viability and is designed for use in Fed-batch applications in conjunction with EX-CELL® Advanced Feed 1 supporting superior performance on all common industrial Chinese Hamster Ovary (CHO) and CHOZN® cell lines.
For alternative feeding a combination with Cellvento® 4 Feed can be recommended to boost performance.
The medium is available in liquid and dry powder format
Get unlimited access to:
Trend and Thought Leadership Articles
Case Studies & White Papers
Extensive Product Database
Members-Only Premium Content
Enter your credentials below to log in. Not yet a member of Bioprocess Online? Subscribe today.
Contact Details
ST. LOUIS, April 7, 2015 /PRNewswire/ — Sigma-Aldrich Corporation (NASDAQ: SIAL) today announced that SAFC® Commercial (www.sigma-aldrich.com/safc), the Company’s business unit providing products and services for use in regulated pharmaceutical and biopharmaceutical applications, has launched the EX-CELL® Advanced™ product line. Designed to address the needs of an evolving industry where speed to market is paramount, the new product line provides for increased performance, streamlines regulatory compliance, and offers the supply chain security needed in today’s biopharmaceutical environment.
The first EX-CELL Advanced product is the high-performing batch media system developed for a range of widely used industrial CHO cell lines, including SAFC’s proprietary CHOZN® cell line. During rigorous testing, the EX-CELL Advanced CHO Fed-batch System outperformed other commercially available equivalents by displaying significantly higher titers. Robust scale up and effortless adaptation offers those in biopharmaceutical development the opportunity to get their process up and running quickly and efficiently. Protein quality, another vital consideration in the development process, remains uncompromised.
«At SAFC we recognize our customers’ needs are constantly changing», said Deb Stutz, Director of Biopharm Marketing. «Our team has utilized unique expertise in raw material science, understanding of process knowledge and decades of experience in cGMP manufacturing in development of the new EX-CELL Advanced line. We are delighted to launch this product in response to market needs for new levels of speed and performance, while continuing to provide the confidence of a secure and transparent supply chain.»
EX-CELL Advanced products are available now as catalog products in a range of pack sizes. To learn more or to make a purchase inquiry about the EX-CELL Advanced CHO Fed-batch System, visit www.sigma-aldrich.com/CHOperformance.
About SAFC: SAFC Commercial, the custom manufacturing and services business unit of Sigma-Aldrich Corporation, is recognized as a top 10 global specialty chemicals and biologics supplier. As a trusted manufacturer for the life science and high-technology industries, SAFC works closely with customers to resolve development challenges and accelerate the product pipeline using its global «Centers of Excellence» and dedicated manufacturing facilities. Its rich portfolio includes high-purity inorganic materials for high-technology applications, critical raw materials and extensive biologics safety testing services for biopharmaceutical manufacturing, and complex, high-potent APIs and key intermediates for pharmaceutical manufacturing. For more information, visit www.sigma-aldrich.com/safc.
About Sigma-Aldrich: Sigma-Aldrich, a leading Life Science and Technology company focused human health and safety, manufactures and distributes 250,000 chemicals, biochemicals and other essential products and 46,000 equipment products to more than 1.4 million customers globally in research and applied labs as well as in industrial and commercial markets. With three distinct business units — Research, Applied and SAFC Commercial — Sigma-Aldrich is committed to enabling science to improve the quality of life. The Company operates in 37 countries, has approximately 9,000 employees worldwide and had sales of $2.79 billion in 2014. For more information about Sigma-Aldrich, please visit its website at www.sigma-aldrich.com.
©2015 Sigma-Aldrich Co. LLC. All rights reserved. Sigma-Aldrich, SAFC, EX-CELL, EX-CELL Advanced and CHOZN are trademarks of Sigma-Aldrich Co. LLC or its affiliates, registered in the U.S. and other countries.
SOURCE Sigma-Aldrich Corporation
Related Links
http://www.sigma-aldrich.com

Ready-for-use Solutions
Xell offers a range of chemically-defined, high-performance, off-the-shelf culture media and feeds for various mammalian cell lines and applications.
All our state-of-the-art cell culture media and feed products are distinguished as follows:
- 100% chemically defined: highest grade of raw materials, carefully monitored
- 100% animal origin-free, serum-free and hydrolysate-free
- Suitable and scalable: from R&D to large-scale GMP production
- Designed as all-in-one solutions: thawing, expansion and production
- Little or no adaption required from other serum-free media
- Developed for long-term suspension cultivation
- Optimized for either stable or transient production
- Robust performance in batch, fed-batch and perfusion cultivations
- Available as liquid and powder formulations
In case you cannot find a suitable medium or feed for your application or cell, reach out, we are always open for developing customized solutions.
Visit webshop
Contact us
CHO cell lines are the most prevalent mammalian expression system in the biopharmaceutical industry. Frequently produced biotherapeutics range from monoclonal antibodies (mAbs) and recombinant proteins, to hormones, enzymes and many more. CHO cells can be used for both stable expression and transient expression. Based on diverse host and production clones, Xell has developed a CHO cell culture medium and feed platform that supports highly efficient protein expression and the individual characteristics of the various clones.
Xell’s CHO media and feeds are developed for long-term, high-performance suspension cultures. The media and feeds are suitable for research, as well as routine large-scale GMP manufacturing.
In order to select the right medium and feed supplements for your CHO clone, please contact us or download our brochure.
- TCX6D | Cell culture medium Order No. 1070
Growth and expression medium for various CHO cell lines.
Find out more
| Protocol for use - TCX10D | Cell culture medium Order No. 1100
Growth and expression medium for CHO GS cell lines.
Find our more
| Protocol for use - TC-42 | Cell culture medium Order No. 510
Growth and expression medium for various CHO cell lines.
Find our more
| Protocol for use | Reference - CHOlean | Cell culture medium Order No. 1140
Lean and robust growth and transfection medium for various CHO clones.
Find out more
| Protocol for use
| Product flyer
- CHO TF | Cell culture medium Order No. 886
Growth and transfection medium for various CHO cell lines.
Find out more
| Protocol for useAlso available for SILAC applications (w/o lysine and arginine)
CHO TF SILAC
- TCX7D | Cell culture feed Order No. 1080
Advanced feed supplement for CHO fed-batch processes. Especially well suited for CHO GS cells.
Find out more
| Protocol for use
HEK
Cell Culture Media and Feed Supplements
Human embryonic kidney (HEK) cells and other human cell lines are commonly used for stable expression and transient expression of recombinant proteins for research and therapeutic use. The well-established mammalian expression system, HEK293, is also widely used for the production of Adeno-associated viruses (AAV) and lentiviruses, viral vector production, as well as for gene therapy applications.
Xell’s HEK cell culture media and feeds are developed for long-term, high-performance suspension culture. The media and feeds are suitable for research, as well as routine large-scale GMP manufacturing.
In order to select the right medium and feed supplements for your HEK application, please contact us or download our brochure.
- HEK GM | Cell culture medium Order No. 851
Growth and production medium for various human cell lines e.g., HEK293.
Find out more | Protocol for use | Reference 1 | Reference 2 - HEK Media Bundle Order No. 841
Media bundle for testing, identifying and selecting the best suitable serum-free, animal-component-free and chemically defined cell culture HEK media for your individual requirements and process
Find out more
- HEK TF | Cell culture medium Order No. 861
Growth medium that supports efficient transfection of human cell lines e.g., HEK293.
Find out more | Protocol for use | Reference | Reference 1 | Reference 2 | Reference 3 | Reference 4Also available for SILAC applications (w/o lysine and arginine)
HEK TF SILAC - HEK ViP NB | Cell culture medium Order No. 891
Transfection and production medium with a basic nutrient level optimized for protein, viral vector and virus production (e.g., AAV and lentivirus).
Find out more | Protocol for use | Product flyer - HEK ViP NX | Cell culture medium Order No. 892
Transfection and production medium with a high nutrient level optimized for protein, viral vector and virus production (e.g., AAV and lentivirus).
Find out more | Protocol for use | Product flyer | Technical note - HEK Media Bundle Order No. 841
Media bundle for testing, identifying and selecting the best suitable serum-free, animal-component-free and chemically defined cell culture HEK media for your individual requirements and process
Find out more
- HEK FS | Cell culture feed Order No. 871
Feed supplement for boosting fed-batch process yields of human cell cultures e.g., HEK293.
Find out more | Protocol for use | Product flyer | Reference 1 | Reference 2 |
Hybridoma
Cell Culture Media and Feed Supplements
Hybridomas are hybrid cell lines formed by fusion of a B cell (e.g., of mouse-origin) with an immortal B myeloma cell. Each hybridoma is able to constitutively produce one specific target monoclonal antibody (mAb). The mAbs that are produced are chemically identical, which qualifies them for various in vivo applications, such as diagnostics, therapeutics, targeted biologics, etc.
Xell’s Hybridoma cell culture media and feeds are optimized for the demanding metabolism of hybridoma cells, such as human, mouse and rat hybridomas and myelomas. The media and feeds are suitable for research, as well as the routine large-scale GMP manufacturing of mAbs.
In order to select the right medium and feed supplements for your Hybridoma application, please contact us or download our brochure.
- Hybridoma Base medium | Cell culture medium Order No. 0027B
Growth and mAbs production medium suitable for various hybridoma cell lines.
Find out more | Protocol for use | Product flyer - HYB GM | Cell culture medium Order No. 890
Lean high-performance medium for mAbs production in various hybridomas cells.
Find out more | Protocol for use | Product flyer
- HYB FS | Cell culture feed Order No. 895
Feeding solution for mAbs production in hybridoma fed-batch processes.
Find out more | Protocol for use
Cell Culture Media and Feeds for BHK and MDxK Cells
Baby Hamster Kidney (BHK) cells are one of the most commonly used mammalian expression system for the production of biopharmaceuticals, recombinant proteins (e.g., Factor VIII) and vaccines (e.g., against SBV). BHK cells can be used for both, transient and stable expression.
Madin-Darby Canine/Bovine Kidney (MDCK/MDBK) are mammalian cell lines widely used in biological and biomedical research as well as in virus and vaccine production.
Xell offers cell culture media and feeds for BHK and MCBK/MDCK cells, optimized for the specific needs of each cell line. The cell culture media and feed solutions are suitable for research and routine large-scale GMP manufacturing.
In order to select the right medium and feed supplements for your application, please contact us or download our brochure.
- BHK Medium | Cell culture medium Order No. 910
Growth and production medium for BHK cell lines.
Find out more | Protocol for use - BHK FS | Cell culture feed Order No. 915
Feed supplement for boosting fed-batch process yields of BHK cell cultures.
Find out more | Protocol for use
- MDXK Medium | Cell culture medium Order No. 1010
Growth and production medium for MDBK and MDCK cell lines.
Find out more | Protocol for use | Reference
We use cookies on our website to give you the most relevant experience by remembering your preferences and repeat visits. By clicking “Accept”, you consent to the use of ALL the cookies.