In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
There are some key words to watch for when reading or writing a word equation. The words «and» or «plus» mean one chemical and another are both reactants or products. The phrase «is reacted with» indicates the chemicals are reactants. If you say «forms», «makes», or «yields», it means the following substances are products.
When you write a chemical equation from a word equation, the reactants always go on the lefthand side of the equation, while the reactants are on the righthand side. This is true even if the products are listed before the reactants in the word equation.
Key Takeaways: Word Equations
- A word equation is an expression of a chemical reaction or mathematical equation using words rather than letters, numbers, and operators.
- In chemistry, a word equation indicates the order of events of a chemical reaction. The number of moles and types of reactants yield the number of moles and types of products.
- Word equations help in learning chemistry because they reinforce the thought process involved in writing a chemical reaction or equation.
Word Equation Examples
The chemical reaction 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as:
hydrogen gas + oxygen gas → steam
As a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen.»
While a word equation doesn’t ordinarily include numbers or symbols (Example: You wouldn’t say «Two H two and one O two makes two H two O», sometimes it is necessary to use a number to indicate the oxidation state of a reactant so that a person writing a chemical equation can do it correctly. This is mostly for the transition metals, which can have multiple oxidation states.
For example, in the reaction between copper and oxygen to form copper oxide, the chemical formula of copper oxide and the number of copper and oxygen atoms involved depends on whether copper(I) or copper(II) participates in the reaction. In this case, it would be fine to say:
copper + oxygen → copper(II) oxide
or
Copper reacts with oxygen to produce copper two oxide.
The (unbalanced) chemical equation for the reaction would start out as:
Cu + O2 → CuO
Balancing the the equation yields:
2Cu + O2 → 2CuO
You would get a different equation and product formula using copper(I):
Cu + O2 → Cu2O
4Cu + O2 → 2Cu2O
More examples of word reactions include:
- Chlorine gas reacts with methane and carbon tetrachloride to produce hydrogen chloride.
- Adding sodium oxide to water produces sodium hydroxide.
- Iodine crystals and chlorine gas react to make solid iron and carbon dioxide gas.
- Zinc and lead two nitrate make zinc nitrate and lead metal.
which means: Zn + Pb (NO3)2 → Zn(NO3)2 + Pb
Why Use Word Equations?
When you’re learning general chemistry, work equations are used to help introduce the concepts of reactants, products, the direction of reactions, and to help you understand precision of language. They may seem annoying, but are a good introduction to the thought processes required for chemistry courses. In any chemical reaction, you need to be able to identify the chemical species that react with each other and what they make.
Word Equations in Other Sciences
Chemistry isn’t the only science to use equations. Physics equations and mathematical equations may also be expressed in words. Usually in these equations two statements are set to be equal to each other. For example, if you way «force equals mass multiplied by acceleration» then you are providing the word equation for the formula F = m*a. Other times, one side of the equation may be less than (<), greater than (>), less than or equal to, or greater than or equal to the other side of the equation. Addition, subtraction, multiplication, division, logs, square roots, integrals, and other operations can be stated in word equations. However, complex equations that contain parentheses to describe the order of operations are very hard to understand as word equations.
Source
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (December 14, 2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
Microsoft Word is a great tool for formatting text, but what if you want to insert a chemistry formula in your Word document?. You can type all types of Chemical reaction in word by going into Insert-> Equation. Whoever has written chemical equation by this way knows how painful it is. But don’t worry you have come to right place. Here you will learn all the shortcuts, tips and tricks required to type Chemical reaction in word, reaction arrows, text above, below arrows and all those that you require for typing Chemical Reaction in Word.
The method that I am talking is called Math Autocorrect which was introduced in Ms Word. This method is very much like LaTeX with some minor changes. By using this method you can type chemical reaction in Microsoft Word around 10 time faster than normal method of using Graphical User Interface (GUI) of Ms Word.
First step of writing equation in Ms Word is to get equation editor. Shortcut for getting equation editor is “Alt”+”=”.
Shortcut for typing Chemical reaction or Chemical Equation:
Writing any chemical equation or chemical formula in Ms Word is very easy when you know shortcut for writing subscript and superscript.
Shortcut of writing subscript
Equation editor shortcut for writing subscript in Ms Word is simply _ (underscore). Any thing following underscore will be converted into subscript automatically after pressing space.
Shortcut of writing subscript
| To get this | Type this in Equation editor (<sp> means pressing space bar) |
| H^+<sp> | |
| H_(2 AfterSpace)<sp> | |
| H_2<sp>SO_4^<sp> |
Similar to subscript, Ms Word has shortcut for writing superscript which is ^ sign. Any thing following ^ sign will be converted automatically into superscript after pressing space
| To get this | Type this in Equation editor (<sp> means pressing space bar) |
| H^+<sp> | |
| H_(+ AfterSpace)<sp> | |
| H_2<sp>SO_4^2-<sp> |
Shortcut for chemical arrows
Arrows are very important part of Chemical reaction. Is signifies direction of reaction and many more things. Shortcut for all the reaction arrows like equilibrium arrow, reversible arrow etc are listed in following table.
| To get this | Type this in Equation editor (<sp> means pressing space bar) |
| rightarrow<sp> or -> | |
| leftarrow<sp> | |
| lrhar<sp> | |
| leftrightarrow<sp> | |
| Type 21c4 and the press “Alt”+”x” | |
| uparrow<sp> | |
| downarrow<sp> |
Text above arrow or Text below arrow
At many place we need to type text above and below arrow to show catalyst, reaction conditions etc. To write text above arrow use above(<text above goes here>) and to write text below arrow use below(<text below goes here>). Following table shows example to do this.
| To do this | Type this in Equation editor (<sp> means pressing space bar) | Comment |
| rightarrowabove(Ni)<sp> | Text above arrow | |
| rightarrowbelow(P=10 MPa)<sp> | Text below arrow | |
| rightarrowbelow(P=10 MPa)above(Ni) or rightarrowabove(Ni) below(P=10 MPa) <sp> |
See Tip given below |
Tip: To make sure that size of arrow fits text length, first type the longest text above or below arrow and press space then type another text below/above. Example here long text in below arrow so we use the following shortcut
rightarrowbelow(P = 10Mpa)<sp>above(Ni)<sp>
Combining all the shortcut into one example. Hydrogen reacts with oxygen even below its lower flammability (4% V/V) limit in presence of catalyst like Pt and Pd. This reaction could be written as
To get this:
Type this: 2H_2<sp>O<sp>+O_2<sp>rightarrowbelow(Energy)<sp>above(Pt)<sp>2H_2<sp>O<sp>, You may include additional spaces in between chemical reaction if required.
Thanks for reading this blog. I would like to hear from you. If you have any comments and suggestion, please write them in comments so that I can improve upon or provide relevant content.
Related Pages
Writing Ionic Equations
Molar Volume, Avogadro’s Law
Chemistry Lessons
Chemical Equation
A chemical equation shows the overall change of reactants to products in a chemical reaction.
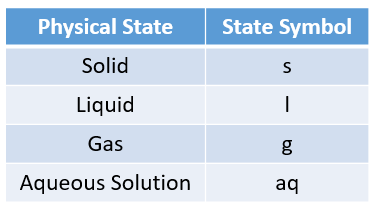
Sometimes, state symbols are required to indicate the physical states of the substances in a chemical
reaction.
The following table gives the physical states and the state symbols used in chemical equations:
solid, liquid, gas, aqueous.
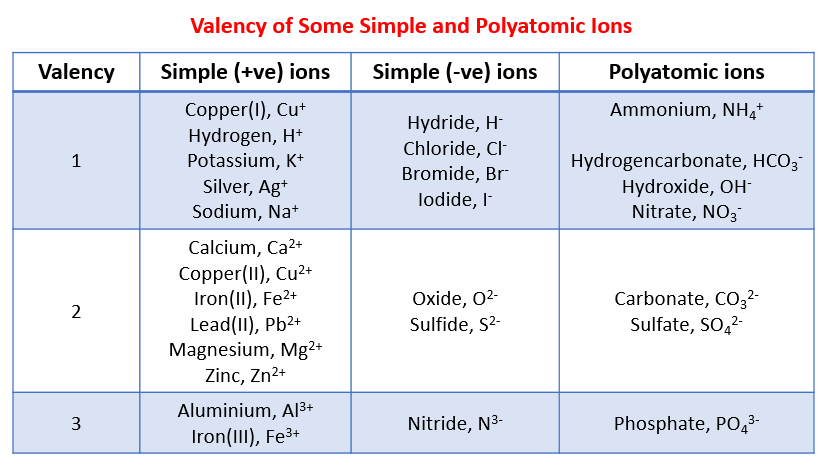
The following table gives the valency of some common ions. This table can be used to help you work
out the chemical formula of the reactants and products.
Here are some simple covalent formulas that you will find useful to remember:
- Water H2O,
- Carbon Dioxide CO2,
- Ammonia NH3,
- Hydrogen H2,
- Oxygen O2,
- Nitrogen N2,
- Sulfur Dioxide or Sulphur Dioxide SO2,
- Methane CH4
Here are some simple ionic formulas that you will find useful to remember:
- Sodium Chloride NaCl,
- Calcium Chloride CaCl2,
- Magnesium Oxide MgO,
- Hydrochloric Acid HCl,
- Sulfuric Acid or Sulphuric Acid H2SO4,
- Nitric Acid HNO3,
- Sodium Hydroxide NaOH,
- Potassium Hydroxide KOH,
- Calcium Hydroxide Ca(OH)2,
- Calcium Carbonate CaCO3,
- Aluminum Oxide Al2O3,
- Iron Oxide Fe2O3
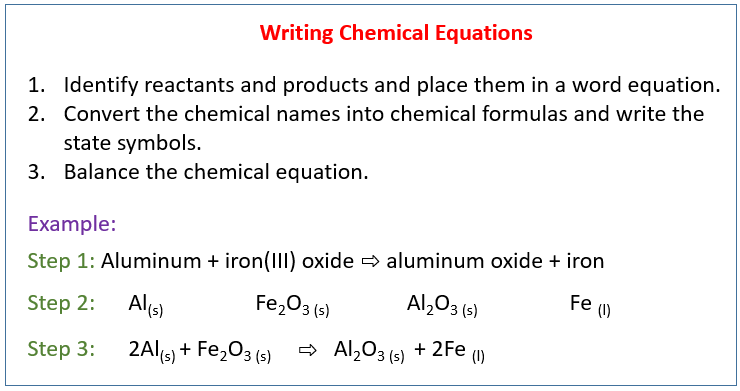
The following diagram shows how to write a chemical equation. Scroll down the page for more examples and solutions.
Conversion Of Word Equation To Chemical Equation
Example:
In a precipitation reaction, sodium hydroxide solution is mixed with iron(II) chloride solution.
Sodium Chloride solution and insoluble iron(II) hydroxide are produced. Write a balanced chemical
equation including the state symbols.
Solution:
Step 1: Identify reactants and products and place them in a
word equation.
sodium hydroxide + iron(II) chloride → sodium chloride + iron(II) hydroxide
Step 2: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 3: Balance the chemical equation.
2NaOH(aq) + FeCl2(aq) → 2NaCl(aq) + Fe(OH)2(s)
Example:
Write a balanced chemical equation for
Sodium(s) + hydrochloric acid(aq) → sodium chloride(aq) + hydrogen(g)
Solution:
Step 1: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 2: Balance the chemical equation.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
How To Write A Balanced Chemical Equation From A Word Equation?
When compounds react, they are chemically changed into new compounds. Every chemical change can be
communicated symbolically using a chemical equation. Chemical equations combine formulas with other
symbols to show what changes takes place.
Examples:
Aluminum + Iron(III) oxide → Aluminum oxide + Iron
Oxygen + Hydrogen → Water
Methane + Oxygen → Carbon Dioxide + Water
Butane + Oxygen → Carbon Dioxide + Water
-
Show Video Lesson
How To Interpret Chemical Symbols And Write Simple Balanced Equations?
Each element is represented by a different symbol.
All these symbols are in the periodic table.
We can use these symbols to show molecules of compounds, and they can show us the ratio of the
different elements which combine to form compounds.
-
Show Video Lesson
Practice Writing Chemical Equations From Word Problems And Balancing Equations
Examples:
- Ammonium nitrate decomposes explosively to form nitrogen, oxygen, and water vapor.
- Dinitrogen tetrahydride reacts with oxygen to produce nitrogen and water.
- Lead(II) nitrate reacts with sodium iodide to create lead (II) iodide and sodium nitrate.
- Phosphorous reacts with oxygen gas to produce diphosphorous pentoxide.
- When calcium comes in contact with water, calcium hydroxide and hydrogen gas is produced.
- When hexane (C6H24) reacts with oxygen a combustion reaction occurs. This reaction produces carbon dioxide and water.
- Sodium hydroxide reacts with iron (III) nitrate to create a precipitate of iron (III) hydroxide in a solution of sodium nitrate.
- Mercury (II) oxide decomposes to produce mercury and oxygen.
- Zinc hydroxide reacts with phosphoric acid (H3PO4) to produce zinc phosphate and water.
- Sulfur dioxide and oxygen combine to produce sulfur trioxide.
- Show Video Lesson
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
If you are using MS Word 2007 or newer, use the equation feature. It is designed for math but works okay for chemistry.
Go to the insert tab. (For shortcut you can press Alt+= sight together)
Click on the equation button on the far right.
Type in your equation. Use the buttons in the ribbon to do superscripts and subscripts. Alternatively you can use _ for subscript and ^ for superscript. The default is to have letters italicized (as variables), so you will want to fix that.
There are also shortcut commands to render most the common things you want. For example, underscore _ creates a subscript and a caret ^ creates a superscript Shortcut for typing subscript and superscript in MS Word 2007|2010|2013|2016 and office 365 . You have access to a wide range of arrows from a pull-down menu, but -> will give you a simple right arrow (although it is not very long). This feature on Word will also accept some (but not all) tex commands for formatting equations.
To get a long arrow, click on the operator but and choose the arrow with the word «yields» written over it under common operator structures. For up arrow and down arrow showing gas liberation and precipitation use uparrow or downarrow followed by space Shortcut for typing arrows of chemical equation in Word 2007 and above.
Click on the word «yields» and replace it with as many spaces as you need to create an arrow of whatever length you want. Shortcut for other types of arrows is.
Finally, finish your equation.
If you need to type above or below arrow just type «above(text above arrow goes here)[space]».Similarly tying below arrow just type below(test below goes here)[space]». How to type chemical equation and arrows in Word 2007 and above.
For older versions of MS Word, go to the insert menu and click on the equation, which launches the Equation Editor Program (you can also find this program on your computer by searching for eqnedt.exe), which gives you the same ability to create equations.
Presentation on theme: «(2.3) CHEMICAL EQUATIONS (p144-145). Recall: Word Equation A word equation — states what reacts and what is produced — uses words instead of formulas.»— Presentation transcript:
1
(2.3) CHEMICAL EQUATIONS (p144-145)
2
Recall: Word Equation A word equation — states what reacts and what is produced — uses words instead of formulas to describe a chemical reaction
3
Word Equation-Examples ex 1: magnesium + oxygen magnesium oxide what reacts what is produced (reactants) (products)
4
Word Equation-Examples ex 2: zinc + hydrochloric acid hydrogen + zinc chloride what reacts what is produced (reactants) (products)
5
Chemical Equation A chemical equation uses chemical symbols and formulas to represent the reactants and products in a chemical reaction
6
Chemical Equation From the word equation you can now write a chemical equation: ex: a) sodium chloride sodium + chlorine (word equation) b) NaCl Na + Cl 2 (chemical equation)
7
Chemical Formulas The chemical formulas of most elements are written simply as the symbol in the periodic table ex: magnesium, Mg sodium, Na But there are some elements that contain more than one atom. For a list, see Table 2.9 p147
8
Chemical Formulas Textbook p 147
9
CLASSWORK Copy Table 2.9 (pg. 147) into your notes pg.147 “Learning Check” # 1 — 4
10
HOMEWORK “Chemical Reactions and Chemical Equations” Worksheet