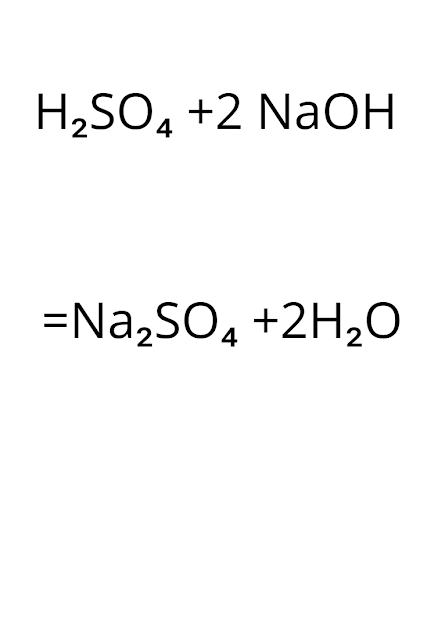Continue Learning about Chemistry
What is the word and chemical equation of calcium oxide plus hydrochloric acid?
Ca2o2 + h2cl
What is the word equation for hydrochloric acid and sodium?
Sodium + Hydrochloric acid —> Sodium Chloride
What is the word equation for hydrochloric acid and aluminum?
The answer is:
Hydrochloric Acid + Aluminium Hydroxide —> Water + Aluminium
Chloride
What is the word equation for hydrochloric acid?
A word equation represent the reactions between metals and
acids. The reaction for zinc and hydrochloric acid would be, zinc
plus hydrochloric acid produces hydrogen plus zinc chloride.
What is the word equation for the reaction between limestone and hydrochloric acid?
Limestone is calcium carbonate(CaCO3).
CaCO3 + 2HCl ——-> CaCl2 + H2O + CO2
【】What is the word equation for the reaction between hydrochloric acid and calcium hydroxide?
Answer:
Calcium hydroxide +Hydrochloric acid ——->Calcium chloride + Water
is the word equation for the reaction between hydrochloric acid and calcium hydroxide.
Explanation:
Calcium hydroxide,Ca(OH)2, is a base and hydrochloric acid,HCl, is an acid.So acid-base reaction will happen and we will get salt .
The following equation is the chemical reaction between hydrochloric acid and calcium hydroxide.
Ca(OH)₂ + HCl —> CaCl₂ + H₂O
Next
Popular posts from this blog
【】How Many Valence Electrons Does Selenium (Se) Have?|| Selenium (Se) Valence Electrons
Selenium has six valence electrons. Read:CO2 Lewis Structure Explanation: Now I am going to show you how to find valence electrons in selenium in just 5 steps. Add caption Step-1: First, find the atomic number of selenium from periodic table. From periodic table ,we see that the atomic number of selenium is 34. Step-2: We know that the atomic number of selenium is 34.So selenium has 34 protons and 34 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Read:CO2 Lewis Structure Step-3: Now write the electron configuration of selenium . Se(34)=1s²2s²2p⁶3s²3p⁶4s²3d¹º4p⁴ Rewrite this from low to high value of n. Se(34)=1s²2s²2p⁶3s²3p⁶ 3d¹º 4s² 4p⁴ Read:CO2 Lewis Structure Step-4: Se(34)=1s²2s²2p⁶3s²3p⁶ 3d¹º 4s² 4p⁴
【●】H2SO4 + NaOH ||Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH)—What is the reaction between sulfuric acid and sodium hydroxide?
Sulfuric acid and Sodium hydroxide Hello,you are in right place to know about the reaction between sulfuric acid and sodium hydroxide. Sulfuric acid is an acid because it has hydrogen ion.The formula for sulfuric acid is H₂SO₄.It is a strong acid. Sodium hydroxide is a base because it has hydroxide ion.The chemical formula for sodium hydroxide is NaOH.It is a strong base. Read this:CO Lewis Structure Read:CO2 Lewis Structure Sulfuric acid(H2SO4) and Sodium hydroxide(NaOH) Acid reacts with base to form salt and water.This type of reaction is called acid base neutralization reaction. In case of sulfuric acid and sodium hydroxide,acid-base neutralization reaction will happen. Sulfuric acid reacts with sodium hydroxide and produce sodium sulphate(Na₂SO₄) and water(H₂O).Here sodium sulphate(Na₂SO₄) is a salt beacause its positive part has come from base and negative part has come from acid. This is a balanced equation: H₂SO₄
【 】Hydrochloric Acid and Sodium Hydroxide||Balanced Equation for Hydrochloric acid and Sodium Hydroxide
Hydrochloric Acid and Sodium Hydroxide Hello,you are in right place to know about the reaction between hydrochloric acid and sodium hydroxide. Hydrochloric acid is an acid because it has hydrogen ion.The formula for hydrochloric acid is HCl.It is a strong acid. Sodium hydroxide is a base because it has hydroxide ion.The chemical formula for sodium hydroxide is NaOH.It is a strong base. Hydrochloric acid (HCl) and Sodium hydroxide(NaOH) Acid reacts with base to form salt and water.This type of reaction is called acid base neutralization reaction. In case of hydrochloric acid and sodium hydroxide,acid-base neutralization reaction will happen. Hydrochloric acid reacts with sodium hydroxide and produce sodium chloride(NaCl) and water(H₂O).Here sodium chloride(NaCl) is a salt beacause its positive part (Na+)has come from base and negative part(Cl-) has come from acid. HCl+ NaOH =NaCl +H₂O Hydrochloric Acid and Sodium Hydroxide Balanced Equation Now I will balance hydrochlor
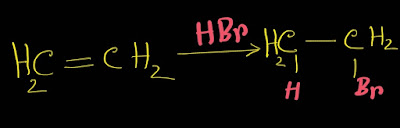
【】How to Convert Ethene to Ethanoic Acid or Acetic Acid||How Ethanoic Acid Is Prepared from Ethene
Answer: To convert ethene to ethanoic acid,we will adopt four steps: Step-1: Ethene reacts with HBr and we get 1-bromo ethane. Step-2: 1-bromo ethane reacts with aqueous KOH and we get ethanol. Step-3: Ethanol is oxidized by K₂Cr₂O₇ plus H₂SO₄ and we get ethanal. Step-4: Again,ethanal is oxidized by K₂Cr₂O₇ plus H₂SO₄ and we get ethanoic acid or acetic acid. Now see all the steps at a glance. How to convert ethene to ethanoic acid Read:CO2 Lewis Structure Next Recommended Posts: ● How to draw lewis structure ● SO2 Lewis Structure ◆ CO Lewis Structure ◆ CO2 Lewis Structure ◆ How to find percentage
【】How to Convert propene to propyne and propyne to propene conversion
How to Convert Propyne to Propene Propyne reacts with hydrogen (H₂) in presence of Pd and BaSO₄ at 25ºc temperature and produce propene(CH₃-CH=CH₂). Here BaSO4 acts as catalyst poison towards Pd catalyst reducing it’s catalytic action and controls the upto first stage. How to Convert Propene to Propyne Step-1: Propene(CH₃-CH=CH₂) reacts with Br₂ in presence of CCl₄ and produce 1,2-dibromo propane( CH₃-CH(Br)CH₂Br). Step-2: 1,2-dibromo propane( CH₃-CH(Br)CH₂Br) reacts with (alc)KOH and produce 1-bromo propene-1(CH₃-CH=CH-Br). Step-3: 1-bromo propene-1(CH₃-CH=CH-Br) reacts with sodamide and produce propyne. Next ● How to find percentage
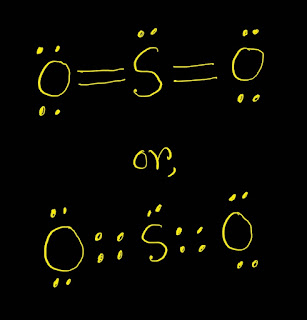
【 】SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
SO2 Lewis Structure Answer: Here is the definition of SO2 Lewis structure. SO2 Lewis structure (sulfur dioxide electron dot structure) is that type of diagram where we show the total 18 valence electrons of SO2 as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●]. In SO2 Lewis structure,there are two double bonds that are going from the atom sulphur to oxygens. In SO2 Lewis structure ,there are totally five lone pairs of electrons.The each oxygen has two lone pairs of electrons and the sulphur atom has one lone pair of electrons. In SO2 Lewis structure ,the oxygen atoms follow the octet rule but the sulfur atom don’t follow the octet rule.It has more than eight electrons. In SO2 Lewis structure,we get nine pairs of electrons.Out of nine pairs of electrons,SO2 has four bond pairs of electrons and five lone pairs of electrons.Here,each oxy
What is the sulfur(S)Electron Configuration?
Full Ground state electron Configuration for Sulfur,S: 1s² 2s² 2p⁶ 3s² 3p⁴ ●Abbreviated Electron Configuration for Sulfur : [Ne] 3s² 3p⁴ . ● Shorthand Electron Configuration for Sulfur,S: [Ne] 3s² 3p⁴ ●Long thand Electron Configuration for Sulfur,S: 1s² 2s² 2p⁶ 3s² 3p⁴ ●Una bbreviated Electron Configuration for Sulfur ,S: 1s² 2s² 2p⁶ 3s² 3p⁴ ●Orbital Diagram of Sulfur,S: Orbital Diagram of Sulfur,S: ● Electron Configuration for a Neutral Sulfur ,S: 1s² 2s² 2p⁶ 3s² 3p⁴ Electron Configuration Chart of Sulphur Explanations: Here is the way you can follow to write the electron configuration og sulphur(S) in just 5 steps. Step-1: To do electron configuration of sulphur, we have to know the atomic number of sulpur(S) . The atomic number of sulphur is 16.So sulpur has 16 electrons and 16 protons. Electron configuration Look how I find the electron configuration for sulphur . This process is applicable for all the elements of the pe
【 】Sodium Hydroxide and Sulfuric Acid Balanced Equation||Balanced Chemical Equation for Sulfuric Acid And Sodium Hydroxide
Sodium Hydroxide and Sulfuric Acid Balanced Equation Now I will balance sulfuric acid and sodium hydroxide reaction. Step-1:write the reaction. H₂SO₄ + NaOH ——>Na₂SO₄ +H₂O Step-2:In the left side, we have H SO₄ Na O To balance this reaction means we need to equalize the number of these above atoms and polyatomic ion. Step-3:Now balance Na atom first.In the left hand side,we have one Na but in the right side,we have two Na.So to balance Na ,we need to put 2 before NaOH in the left side. H₂SO₄ +2 NaOH ——>Na₂SO₄ + H₂O Step-3:Now balance H. After step 3 we are getting … H₂SO₄ +2 NaOH ——>Na₂SO₄ + H₂O In the left hand side,we have 4 hydrogens but in the right side ,we have 2 hydrogen’s.So we need to place 2 before H₂O in the right side. So we are getting… H₂SO₄ +2 NaOH ——>Na₂SO₄ +2 H₂O Step-4:Now balance SO4 Ater step 3, we are getting… H₂SO₄ +2 NaOH ——>Na₂SO₄ +2 H₂O In the left side ,we have one SO₄ and in the
【 】CO Lewis Structure ,Valence Electrons ,Formal Charge ,Polar or Nonpolar
CO Lewis Structure Answer: CO Lewis structure(carbon monoxide electron dot structure) is that type of structure where we show the total ten valence electrons of CO as dots , or dots and dashes(-).In Lewis structure,it is common that a bonding pair of two electrons can be shown by dash(-) or dots(●●) but a lone pair of two electrons is shown by dots[●●]. CO Lewis Structure In CO Lewis structure,the carbon atom follows the octet rule and the oxygen atom also follows the octet rule.So,CO follows the octet rule for all the atoms. In CO Lewis structure,we get five pairs of electrons.Out of five pairs of electrons,CO has three bond pairs and two lone pairs of electrons.Here carbon has one lone pair and oxygen has one lone pair of electrons. Explanation: Today we are going to learn to draw CO Lewis structure (Carbon monoxide Lewis structure) in just few steps. CO Lewis Structure CO Lewis Structure Setup Steps There are 5 steps that you need to follow when drawing the C
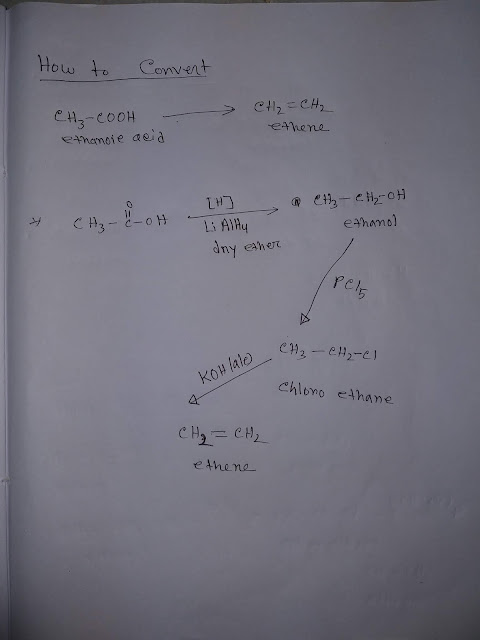
How to Convert Ethanoic Acid to Ethene
Look at the following steps to convert ethanoic acid to ethene. Step-1 : Ethanoic acid is reduced by lithium aluminum hydride in presence of dry ether and we get the product ethanol. Step-2: Ethanol reacts with phosphorus pentachloride and produce chloro ethane. Step-3: Chloro ethane reacts with alcoholic potassium hydroxide and produce ethene. Next How to Draw Lewis Structure for CO2
Home
>471-34-1>Q&A>What is a balanced word and symbol equation to show the reaction between calcium carbonate and hydrochloric acid?
Answer
Follow +1
Napoleon
Answered Jan 19 2022
CaCO3 + 2HCl —-> CaCl2 + CO2 + H2O
Calcium carbonate + hydrocloric acid —-> calcium chloride + carbon dioxide + water
Garrison
Answered Jan 19 2022
Well, calcium carbonate plus hydrochloric acid gives aqueous calcium chloride, and carbon dioxide, and water… And symbolically….
471-34-1 Related Q&A
-
Promoting calcium carbonate decomposition
It is well-known that calcium carbonate undergoes thermal decomposition forming calcium oxide and C O X 2 » style=»position: relative;» tabindex=»0″ id=»MathJax-Element-2-Frame» class=»MathJax» C O X 2 C O X 2 . However, the reaction requires a lot of heat (approximately 840 °C). Is it possible to… -
How much calcium carbonate is too much?
You cannot consume enough. It is not absorbable or bioavailable to humans. It is better as a plant mineral. The molecular bond between the calcium and carbonate molecule is too strong for humans to breakdown in their digestive tract. I estimate you absorb at best 5–6%. Dont waste your money. I sug… -
What is the product of the chemical reaction of carbonic acid and calcium carbonate?
Soluble calcium bicarbonate should be the product. Carbonic acid (H2CO3) is only an aqueous solution of carbon dioxide (CO2 + H2O). When insoluble calcium carbonate is treated with carbonic acid, the former dissolves to give a colourless aqueous solution of calcium bicarbonate. CaCO3 (s) + CO2 + H2… -
How do you separate calcium carbonate and calcium chloride?
Slurry the 2 salts up in water … the carbonate is pretty insoluble … the chloride is water-soluble … pass thru a filter …. and the material retained by the filter is the carbonate …. To access the chloride (AFTER the carbonate has been removed thru several cycles) …. add ethyl alcohol, and th…
Lastest Questions
- What is the difference between hyaluronic acid and niacinamide?
- What makes hydrazine so dangerous?
- How would propargyl bromide react with triethylamine under room temperature, an SN2-reaction or an acid-base reaction?
- Cobalt glass colour of potassium as
Encyclopedia
| Density | 2.93 |
| Melting Point | 825℃ |
| Boiling Point | 333.6°C at 760mmHg |
| Vapour | 0 mm Hg (approx) (NIOSH, 2016) |
| Refractive Index | 1.6583 |
| Flash Point | 197℃ |
| HS Code | 28365000 |
471-34-1 Related Suppilers
-
2YRS
Hebei Yingong New Material Technology Co.,Ltd.
Country/Region:China
Product Name:Factory supply Calcium Carbonate powder CAS 471-34-1
Update Time:Apr 13 2023
Main products:xylazine hcl,phenacetin,4-Methylpropiophenone,(2-Bromoethyl)benzene,4-Methoxybenzoyl chloride
Inquire
-
2YRS
Wuhan Xiju Biotechnology Co., Ltd.
Country/Region:China
Product Name:Cheap and good quality calcium carbonate CAS 471-34-1 in stock
Update Time:Apr 13 2023
Main products:My whatsapp:+86 13043111536 cas no.1451-82-7,79099-07-3,5449-12-7,5337-93-9,49851-31-2,288573-56-8
Inquire
-
2YRS
Hebei Zebo Biotechnology Co., LTD
Country/Region:China
Product Name:Digestive system medication Calcium carbonate 471-34-1
Update Time:Apr 13 2023
Main products:Organic intermediates, Organic solvents,Cosmetic raw materials …
Inquire
-
2YRS
Hebei Crovell Biotech Co. Ltd
Country/Region:China
Product Name:Calcium carbonate 471-34-1 factory
Update Time:Nov 08 2022
Main products:Cosmetic Raw Materials,solvents,etc.
Inquire
Calcium carbonate (CaCO3)
is a metal carbonate compound and reacts with hydrochloric acid (HCl) to
produce carbon dioxide (CO2), calcium chloride (CaCl2) and water. You can see, carbon
dioxide gas is released through the solution.
CaCO3 + HCl → CaCl2 + CO2 + H2O
Carbon dioxide gas is released when a dilute acid is added to a metal carbonate compound.
Metal carbonate compounds react with dilute acids and emit carbon dioxide gas.
Balanced chemical equation of CaCO3 and HCl reaction with physical states
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
Calcium carbonate is not soluble
in water and exists as white precipitate in the water. When aqueous hydrochloric acid is added,
calcium chloride, carbon dioxide and water are formed.
- Calcium chloride (CaCl2) is soluble in water and colorless. So, it exists as an aqueous solution. Therefore,
you can see, white precipitate is dissolved and colorless solution is formed with time. - Also, due to formation of carbon dioxide gas, gas bubbles are rising to the top of the solution.
This reaction is used to calculate purity of CaCO3 samples when they are mixed with impurities.
If impurity material does not react with dilute hydrochloric acid, You can conduct this experiment. Released carbon dioxide
volume is measured and then calculate the released amount (mol) of carbon dioxide gas. Then, you can calculate the reacted
calcium carbonate amount and mass.
Reaction properties
- When calcium carbonate precipitate exist in water, that solution become weak basic due to presence of carbonate ion. When
aqueous HCl is added, carbonate is converted to carbon dioxide and alkalinity of the solution decreases.
Calcium carbonate and hydrochloric acid reaction is an exothermic reaction.
When calcium carbonate reacts with hydrochloric acid, heat is released to the environment.
Questions asked by students
Ask your question and find the
answer free.
When HCl reacts with calcium carbonate why heat is released?
If heat is released from a reaction, it means products of the reactions are more stable than reactants. When something is stable, it should have less energy. When HCl reacts with calcium carbonate, calcium chloride and carbon dioxide gas are given.
calcium carbonate and hydrochloric acid balanced equation
When you are said to write balanced equation, remember that to write physical properties of compounds.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
What happens when CaCO3 reacts with dilute HCl acid?
Calcium carbonate reacts with dilute acids to produce a calcium salt, water and carbon dioxide gas: calcium carbonate +
hydrochloric acid → calcium chloride + water + carbon dioxide.
What does HCl and CaCO3 produce?
Calcium chloride, carbon dioxide and water are given as products.
Can I identify calcium carbonate from zinc carbonate from this reaction?
Both carbonates are not insoluble in water and form white precipitates. Calcium carbonate and zinc carbonate reacts with
dilute hydrochloric acid and emit carbon dioxide gas form colorless solutions. (CaCl2 and ZnCl2 are
colorless solutions).
But, treating hydrogen sulfide gas to calcium chloride and zinc chloride can be used to identify solutions. Calcium sulfide
is soluble in water. But, ZnS is not
a soluble sulfide in water and form a white precipitate.
Related Tutorials
Sodium carbonate and HCl reaction
FeCl2 + NaOH reaction
NaOH and chlorine gas reaction
Solubility
of metal carbonate compounds compounds
Ab Padhai karo bina ads ke
Khareedo DN Pro and dekho sari videos bina kisi ad ki rukaavat ke!
Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams.
welcome to ask about the right correctly balanced equation for the following word equation hair word equation is given that calcium Ka Kitchen with hydrochloric acid chloride water and carbon dioxide first of all write the for calcium + 2 and carbonate is jio 3 carbonate is there you 3 — 2 on combining with calcium calcium carbonate like this year on reaction with hydrochloric acid hydrochloric acid is HCL it is hydrochloric acid on reaction of calcium chloride chloride is real and here calcium is CA + 2 so it form cacl2 compound and
water H2O and also produce hair 80202 is carbon dioxide it is even in the equation to balance the equation for stop all bro and table and write the element on the reactant sites on the reactant side calcium is percent carbon is burnt oxygen is present and hydrogen chlorine is element are present on the reactant size and hear calcium carbon oxygen hydrogen and chlorine also present on production cost of all the number write the number of element on the reactive nitrogen is one carbon is also one of the reason is 3 hydrogen is one and chlorine is also worked on the product side calcium is one carbon is
oxygen is oneplus 2 and hydrogen is 2 and chlorine is also to to balance the chlorine for X 200 chlorine on creatine side is 2 and product side also to so if x is chlorine by 2 then it is multiplied by 2 then hydrogen atom as to get the world to hydrogen as you will be balanced hydrogen is balance and other element is already here so this is the balanced chemical equation and this is the contrary to this is our answer thank you have a nice day