Related Pages
Writing Ionic Equations
Molar Volume, Avogadro’s Law
Chemistry Lessons
Chemical Equation
A chemical equation shows the overall change of reactants to products in a chemical reaction.
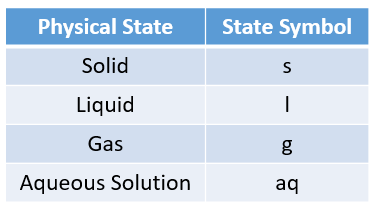
Sometimes, state symbols are required to indicate the physical states of the substances in a chemical
reaction.
The following table gives the physical states and the state symbols used in chemical equations:
solid, liquid, gas, aqueous.
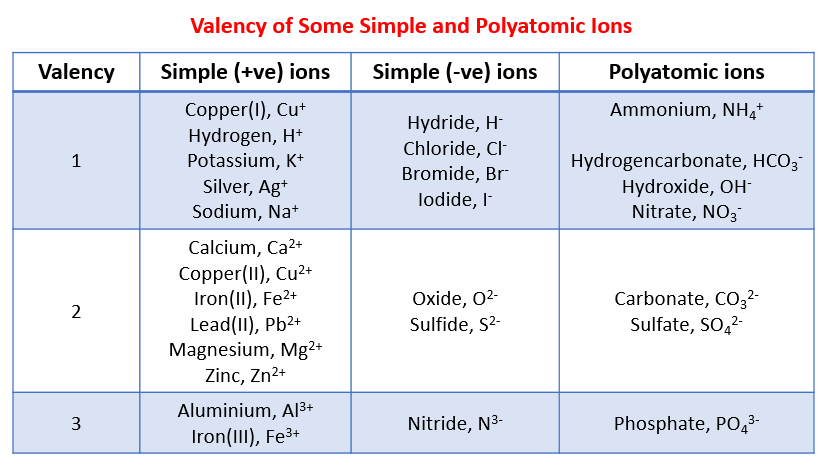
The following table gives the valency of some common ions. This table can be used to help you work
out the chemical formula of the reactants and products.
Here are some simple covalent formulas that you will find useful to remember:
- Water H2O,
- Carbon Dioxide CO2,
- Ammonia NH3,
- Hydrogen H2,
- Oxygen O2,
- Nitrogen N2,
- Sulfur Dioxide or Sulphur Dioxide SO2,
- Methane CH4
Here are some simple ionic formulas that you will find useful to remember:
- Sodium Chloride NaCl,
- Calcium Chloride CaCl2,
- Magnesium Oxide MgO,
- Hydrochloric Acid HCl,
- Sulfuric Acid or Sulphuric Acid H2SO4,
- Nitric Acid HNO3,
- Sodium Hydroxide NaOH,
- Potassium Hydroxide KOH,
- Calcium Hydroxide Ca(OH)2,
- Calcium Carbonate CaCO3,
- Aluminum Oxide Al2O3,
- Iron Oxide Fe2O3
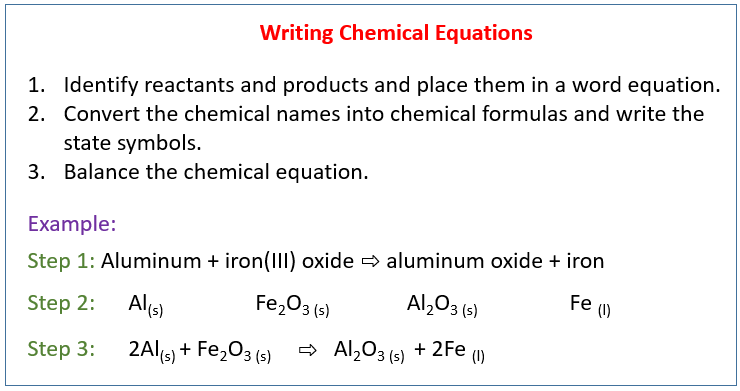
The following diagram shows how to write a chemical equation. Scroll down the page for more examples and solutions.
Conversion Of Word Equation To Chemical Equation
Example:
In a precipitation reaction, sodium hydroxide solution is mixed with iron(II) chloride solution.
Sodium Chloride solution and insoluble iron(II) hydroxide are produced. Write a balanced chemical
equation including the state symbols.
Solution:
Step 1: Identify reactants and products and place them in a
word equation.
sodium hydroxide + iron(II) chloride → sodium chloride + iron(II) hydroxide
Step 2: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 3: Balance the chemical equation.
2NaOH(aq) + FeCl2(aq) → 2NaCl(aq) + Fe(OH)2(s)
Example:
Write a balanced chemical equation for
Sodium(s) + hydrochloric acid(aq) → sodium chloride(aq) + hydrogen(g)
Solution:
Step 1: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 2: Balance the chemical equation.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
How To Write A Balanced Chemical Equation From A Word Equation?
When compounds react, they are chemically changed into new compounds. Every chemical change can be
communicated symbolically using a chemical equation. Chemical equations combine formulas with other
symbols to show what changes takes place.
Examples:
Aluminum + Iron(III) oxide → Aluminum oxide + Iron
Oxygen + Hydrogen → Water
Methane + Oxygen → Carbon Dioxide + Water
Butane + Oxygen → Carbon Dioxide + Water
- Show Video Lesson
How To Interpret Chemical Symbols And Write Simple Balanced Equations?
Each element is represented by a different symbol.
All these symbols are in the periodic table.
We can use these symbols to show molecules of compounds, and they can show us the ratio of the
different elements which combine to form compounds.
- Show Video Lesson
Practice Writing Chemical Equations From Word Problems And Balancing Equations
Examples:
- Ammonium nitrate decomposes explosively to form nitrogen, oxygen, and water vapor.
- Dinitrogen tetrahydride reacts with oxygen to produce nitrogen and water.
- Lead(II) nitrate reacts with sodium iodide to create lead (II) iodide and sodium nitrate.
- Phosphorous reacts with oxygen gas to produce diphosphorous pentoxide.
- When calcium comes in contact with water, calcium hydroxide and hydrogen gas is produced.
- When hexane (C6H24) reacts with oxygen a combustion reaction occurs. This reaction produces carbon dioxide and water.
- Sodium hydroxide reacts with iron (III) nitrate to create a precipitate of iron (III) hydroxide in a solution of sodium nitrate.
- Mercury (II) oxide decomposes to produce mercury and oxygen.
- Zinc hydroxide reacts with phosphoric acid (H3PO4) to produce zinc phosphate and water.
- Sulfur dioxide and oxygen combine to produce sulfur trioxide.
-
Show Video Lesson
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
There are some key words to watch for when reading or writing a word equation. The words «and» or «plus» mean one chemical and another are both reactants or products. The phrase «is reacted with» indicates the chemicals are reactants. If you say «forms», «makes», or «yields», it means the following substances are products.
When you write a chemical equation from a word equation, the reactants always go on the lefthand side of the equation, while the reactants are on the righthand side. This is true even if the products are listed before the reactants in the word equation.
Key Takeaways: Word Equations
- A word equation is an expression of a chemical reaction or mathematical equation using words rather than letters, numbers, and operators.
- In chemistry, a word equation indicates the order of events of a chemical reaction. The number of moles and types of reactants yield the number of moles and types of products.
- Word equations help in learning chemistry because they reinforce the thought process involved in writing a chemical reaction or equation.
Word Equation Examples
The chemical reaction 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as:
hydrogen gas + oxygen gas → steam
As a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen.»
While a word equation doesn’t ordinarily include numbers or symbols (Example: You wouldn’t say «Two H two and one O two makes two H two O», sometimes it is necessary to use a number to indicate the oxidation state of a reactant so that a person writing a chemical equation can do it correctly. This is mostly for the transition metals, which can have multiple oxidation states.
For example, in the reaction between copper and oxygen to form copper oxide, the chemical formula of copper oxide and the number of copper and oxygen atoms involved depends on whether copper(I) or copper(II) participates in the reaction. In this case, it would be fine to say:
copper + oxygen → copper(II) oxide
or
Copper reacts with oxygen to produce copper two oxide.
The (unbalanced) chemical equation for the reaction would start out as:
Cu + O2 → CuO
Balancing the the equation yields:
2Cu + O2 → 2CuO
You would get a different equation and product formula using copper(I):
Cu + O2 → Cu2O
4Cu + O2 → 2Cu2O
More examples of word reactions include:
- Chlorine gas reacts with methane and carbon tetrachloride to produce hydrogen chloride.
- Adding sodium oxide to water produces sodium hydroxide.
- Iodine crystals and chlorine gas react to make solid iron and carbon dioxide gas.
- Zinc and lead two nitrate make zinc nitrate and lead metal.
which means: Zn + Pb (NO3)2 → Zn(NO3)2 + Pb
Why Use Word Equations?
When you’re learning general chemistry, work equations are used to help introduce the concepts of reactants, products, the direction of reactions, and to help you understand precision of language. They may seem annoying, but are a good introduction to the thought processes required for chemistry courses. In any chemical reaction, you need to be able to identify the chemical species that react with each other and what they make.
Word Equations in Other Sciences
Chemistry isn’t the only science to use equations. Physics equations and mathematical equations may also be expressed in words. Usually in these equations two statements are set to be equal to each other. For example, if you way «force equals mass multiplied by acceleration» then you are providing the word equation for the formula F = m*a. Other times, one side of the equation may be less than (<), greater than (>), less than or equal to, or greater than or equal to the other side of the equation. Addition, subtraction, multiplication, division, logs, square roots, integrals, and other operations can be stated in word equations. However, complex equations that contain parentheses to describe the order of operations are very hard to understand as word equations.
Source
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (December 14, 2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.

A balanced chemical equation represents a chemical reaction as chemical formulas and numbers. Here is a collection of more than 10 balanced chemical equations. Use them as homework examples or to review the principles of balancing equations.
Balanced Equation Basics
- Elements are represented using their element symbols.
- The left side of the reaction lists the reactants, the right side lists the products, and the reaction arrow indicates the direction in which the reaction proceeds.
- In a balanced chemical equation, the same number and type of atoms are present on both sides of the reaction arrow.
- The number in front of a chemical formula is its coefficient and is the number of moles of that element or compound. If there is 1 mole of a substance, the number is omitted (e.g., write CO instead of 1 CO).
- Subscripts after an element symbol indicate the number of atoms of the element in a substance. If there is no subscript, it means there is one atom of that element.
- The total number of atoms in a compound is the subscript multiplied by the coefficient (e.g., 4H2O contains 4 x 2 = 8 atoms of hydrogen and 1 x 4 = 4 atoms of oxygen).
Balanced Chemical Equations
6 CO2 + 6 H2O → C6H12O6 + 6 O2 (balanced equation for photosynthesis)
2 AgI + Na2S → Ag2S + 2 NaI
Ba3N2 + 6 H2O → 3 Ba(OH)2 + 2 NH3
3 CaCl2 + 2 Na3PO4 → Ca3(PO4)2 + 6 NaCl
4 FeS + 7 O2 → 2 Fe2O3 + 4 SO2
PCl5 + 4 H2O → H3PO4 + 5 HCl
2 As + 6 NaOH → 2 Na3AsO3 + 3 H2
3 Hg(OH)2 + 2 H3PO4 → Hg3(PO4)2 + 6 H2O
12 HClO4 + P4O10 → 4 H3PO4 + 6 Cl2O7
8 CO + 17 H2 → C8H18 + 8 H2O
10 KClO3 + 3 P4 → 3 P4O10 + 10 KCl
SnO2 + 2 H2 → Sn + 2 H2O
3 KOH + H3PO4 → K3PO4 + 3 H2O
2 KNO3 + H2CO3 → K2CO3 + 2 HNO3
Na3PO4 + 3 HCl → 3 NaCl + H3PO4
TiCl4 + 2 H2O → TiO2 + 4 HCl
C2H6O + 3 O2 → 2 CO2 + 3 H2O
2 Fe + 6 HC2H3O2 → 2 Fe(C2H3O2)3 + 3 H2
4 NH3 + 5 O2 → 4 NO + 6 H2O
B2Br6 + 6 HNO3 → 2 B(NO3)3 + 6 HBr
4 NH4OH + KAl(SO4)2·12H2O → Al(OH)3 + 2 (NH4)2SO4 + KOH + 12 H2O
Balanced Chemical Equations as Word Equations
Sometimes you may be asked to say a balanced chemical equation as a word equation. To read an equation aloud, you need to know the chemical name of the substance. The coefficients are read as “X moles of”, the subscripts aren’t stated because they are implied in the chemical name, and the reaction arrow is read as “yields” or “forms”.
For example, the following equation:
4 NH3 + 5 O2 → 4 NO + 6 H2O
Is read as:
Four moles of ammonia plus five moles of oxygen yields four moles of nitric oxide plus six moles of water.
Check Your Work
When you write a balanced equation, you should check your work to make certain it’s balanced and that it is written in its most-reduced form.
- Count the number of atoms of each element on both sides of the reaction arrow. They should be the same.
- Make certain all elements are included. If an element appears on one side of the reaction, it must also appear on the other side.
- Check to see if you can factor out the coefficients. For example, if all coefficients can be divided by 2, the equation may be balanced, but it could be written as a simpler balanced equation. Ideally, the equation should list the smallest mole ratios of reactants and products.
References
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
- Crosland, M.P. (1959). “The use of diagrams as chemical ‘equations’ in the lectures of William Cullen and Joseph Black”. Annals of Science. 15 (2): 75–90. doi:10.1080/00033795900200088
- Thorne, Lawrence R. (2010). “An Innovative Approach to Balancing Chemical-Reaction Equations: A Simplified Matrix-Inversion Technique for Determining the Matrix Null Space”. Chem. Educator. 15: 304–308.
Presentation on theme: «Balancing Chemical Equations. Writing Word Equations Steps in writing equations: 1.word equation A.types of reactions B.predicting products 2.formula.»— Presentation transcript:
1
Balancing Chemical Equations
2
Writing Word Equations Steps in writing equations: 1.word equation A.types of reactions B.predicting products 2.formula equation (unbalanced) 3.balanced chemical equation 4.net ionic equation (for precipitation reaction)
3
Types of Chemical Reactions
4
1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion A + B → AB AB → A + B AB+ C → AC + B AB+ CD → AD + CB CH+ O 2 → CO 2 + H 2 O (N.B., in a double replacement, cations stay in front)
5
Examples of Chemical Reactions 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion 2H 2 O → 2H 2 + O 2 2Al + 3CuCl 2 → 2AlCl 3 + 3Cu AgNO 3 + NaCl → AgCl + NaNO 3 1C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O 2H 2 + O 2 → 2H 2 O (cellular respiration)
6
Types of Chemical Reactions 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion A + B → AB AB → A + B AB+ C → AC + B AB+ CD → AD + CB CH+ O 2 → CO 2 + H 2 O N.B. A.“replacement” or “displacement” B.in a double replacement, cations stay in front C.subtypes – e.g,. 2NaCl + 1F 2 2NaF + 2Na is a halogen s.r. reaction
7
Steps to completing a balanced chemical equation. Given words of reactants. 1.Determine the type of reaction. 2.Write the words for the products. (even when given the reactants formulas) 3.Translate the words into formulas. 4.Balance 5.Check
8
Ag + CuSO 4 1.Single replacement (cation replacement) 2.Ag + CuSO 4 copper + silver chloride (Silver is +1) 3.Ag + CuSO 4 Cu + AgCl 4.1Ag + 1CuSO 4 1Cu + 1AgCl