In chemistry, a word equation is a chemical reaction expressed in words rather than chemical formulas. A word equation should state the reactants (starting materials), products (ending materials), and direction of the reaction in a form that could be used to write a chemical equation.
There are some key words to watch for when reading or writing a word equation. The words «and» or «plus» mean one chemical and another are both reactants or products. The phrase «is reacted with» indicates the chemicals are reactants. If you say «forms», «makes», or «yields», it means the following substances are products.
When you write a chemical equation from a word equation, the reactants always go on the lefthand side of the equation, while the reactants are on the righthand side. This is true even if the products are listed before the reactants in the word equation.
Key Takeaways: Word Equations
- A word equation is an expression of a chemical reaction or mathematical equation using words rather than letters, numbers, and operators.
- In chemistry, a word equation indicates the order of events of a chemical reaction. The number of moles and types of reactants yield the number of moles and types of products.
- Word equations help in learning chemistry because they reinforce the thought process involved in writing a chemical reaction or equation.
Word Equation Examples
The chemical reaction 2 H2(g) + O2(g) → 2 H2O(g) would be expressed as:
hydrogen gas + oxygen gas → steam
As a word equation or as «Hydrogen and oxygen react to form water» or «Water is made by reacting hydrogen and oxygen.»
While a word equation doesn’t ordinarily include numbers or symbols (Example: You wouldn’t say «Two H two and one O two makes two H two O», sometimes it is necessary to use a number to indicate the oxidation state of a reactant so that a person writing a chemical equation can do it correctly. This is mostly for the transition metals, which can have multiple oxidation states.
For example, in the reaction between copper and oxygen to form copper oxide, the chemical formula of copper oxide and the number of copper and oxygen atoms involved depends on whether copper(I) or copper(II) participates in the reaction. In this case, it would be fine to say:
copper + oxygen → copper(II) oxide
or
Copper reacts with oxygen to produce copper two oxide.
The (unbalanced) chemical equation for the reaction would start out as:
Cu + O2 → CuO
Balancing the the equation yields:
2Cu + O2 → 2CuO
You would get a different equation and product formula using copper(I):
Cu + O2 → Cu2O
4Cu + O2 → 2Cu2O
More examples of word reactions include:
- Chlorine gas reacts with methane and carbon tetrachloride to produce hydrogen chloride.
- Adding sodium oxide to water produces sodium hydroxide.
- Iodine crystals and chlorine gas react to make solid iron and carbon dioxide gas.
- Zinc and lead two nitrate make zinc nitrate and lead metal.
which means: Zn + Pb (NO3)2 → Zn(NO3)2 + Pb
Why Use Word Equations?
When you’re learning general chemistry, work equations are used to help introduce the concepts of reactants, products, the direction of reactions, and to help you understand precision of language. They may seem annoying, but are a good introduction to the thought processes required for chemistry courses. In any chemical reaction, you need to be able to identify the chemical species that react with each other and what they make.
Word Equations in Other Sciences
Chemistry isn’t the only science to use equations. Physics equations and mathematical equations may also be expressed in words. Usually in these equations two statements are set to be equal to each other. For example, if you way «force equals mass multiplied by acceleration» then you are providing the word equation for the formula F = m*a. Other times, one side of the equation may be less than (<), greater than (>), less than or equal to, or greater than or equal to the other side of the equation. Addition, subtraction, multiplication, division, logs, square roots, integrals, and other operations can be stated in word equations. However, complex equations that contain parentheses to describe the order of operations are very hard to understand as word equations.
Source
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (December 14, 2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
Related Pages
Writing Ionic Equations
Molar Volume, Avogadro’s Law
Chemistry Lessons
Chemical Equation
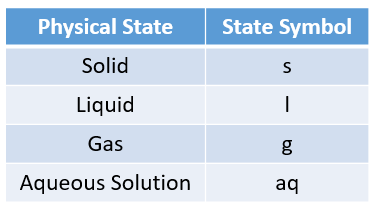
A chemical equation shows the overall change of reactants to products in a chemical reaction.
Sometimes, state symbols are required to indicate the physical states of the substances in a chemical
reaction.
The following table gives the physical states and the state symbols used in chemical equations:
solid, liquid, gas, aqueous.
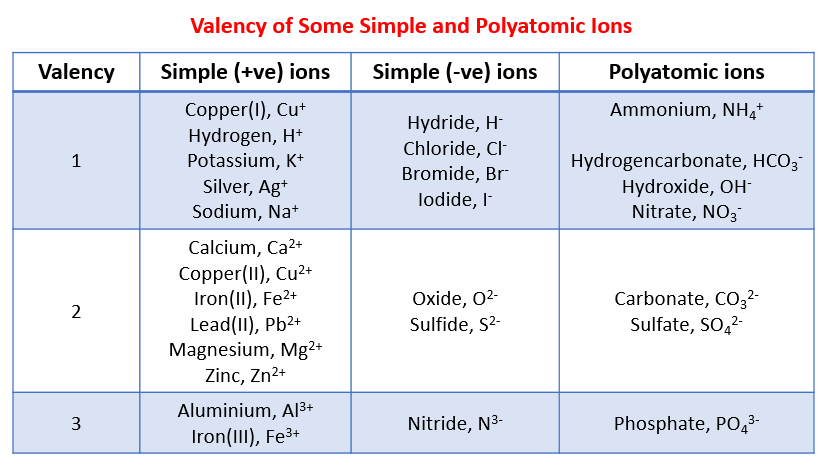
The following table gives the valency of some common ions. This table can be used to help you work
out the chemical formula of the reactants and products.
Here are some simple covalent formulas that you will find useful to remember:
- Water H2O,
- Carbon Dioxide CO2,
- Ammonia NH3,
- Hydrogen H2,
- Oxygen O2,
- Nitrogen N2,
- Sulfur Dioxide or Sulphur Dioxide SO2,
- Methane CH4
Here are some simple ionic formulas that you will find useful to remember:
- Sodium Chloride NaCl,
- Calcium Chloride CaCl2,
- Magnesium Oxide MgO,
- Hydrochloric Acid HCl,
- Sulfuric Acid or Sulphuric Acid H2SO4,
- Nitric Acid HNO3,
- Sodium Hydroxide NaOH,
- Potassium Hydroxide KOH,
- Calcium Hydroxide Ca(OH)2,
- Calcium Carbonate CaCO3,
- Aluminum Oxide Al2O3,
- Iron Oxide Fe2O3
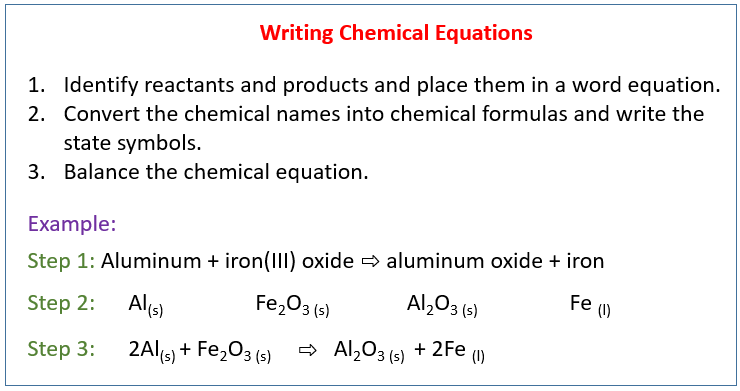
The following diagram shows how to write a chemical equation. Scroll down the page for more examples and solutions.
Conversion Of Word Equation To Chemical Equation
Example:
In a precipitation reaction, sodium hydroxide solution is mixed with iron(II) chloride solution.
Sodium Chloride solution and insoluble iron(II) hydroxide are produced. Write a balanced chemical
equation including the state symbols.
Solution:
Step 1: Identify reactants and products and place them in a
word equation.
sodium hydroxide + iron(II) chloride → sodium chloride + iron(II) hydroxide
Step 2: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 3: Balance the chemical equation.
2NaOH(aq) + FeCl2(aq) → 2NaCl(aq) + Fe(OH)2(s)
Example:
Write a balanced chemical equation for
Sodium(s) + hydrochloric acid(aq) → sodium chloride(aq) + hydrogen(g)
Solution:
Step 1: Convert the chemical names into chemical formulas.
Place them based on the chemical equation and write the state symbols.
Step 2: Balance the chemical equation.
2Na(s) + 2HCl(aq) → 2NaCl(aq) + H2(g)
How To Write A Balanced Chemical Equation From A Word Equation?
When compounds react, they are chemically changed into new compounds. Every chemical change can be
communicated symbolically using a chemical equation. Chemical equations combine formulas with other
symbols to show what changes takes place.
Examples:
Aluminum + Iron(III) oxide → Aluminum oxide + Iron
Oxygen + Hydrogen → Water
Methane + Oxygen → Carbon Dioxide + Water
Butane + Oxygen → Carbon Dioxide + Water
- Show Video Lesson
How To Interpret Chemical Symbols And Write Simple Balanced Equations?
Each element is represented by a different symbol.
All these symbols are in the periodic table.
We can use these symbols to show molecules of compounds, and they can show us the ratio of the
different elements which combine to form compounds.
- Show Video Lesson
Practice Writing Chemical Equations From Word Problems And Balancing Equations
Examples:
- Ammonium nitrate decomposes explosively to form nitrogen, oxygen, and water vapor.
- Dinitrogen tetrahydride reacts with oxygen to produce nitrogen and water.
- Lead(II) nitrate reacts with sodium iodide to create lead (II) iodide and sodium nitrate.
- Phosphorous reacts with oxygen gas to produce diphosphorous pentoxide.
- When calcium comes in contact with water, calcium hydroxide and hydrogen gas is produced.
- When hexane (C6H24) reacts with oxygen a combustion reaction occurs. This reaction produces carbon dioxide and water.
- Sodium hydroxide reacts with iron (III) nitrate to create a precipitate of iron (III) hydroxide in a solution of sodium nitrate.
- Mercury (II) oxide decomposes to produce mercury and oxygen.
- Zinc hydroxide reacts with phosphoric acid (H3PO4) to produce zinc phosphate and water.
- Sulfur dioxide and oxygen combine to produce sulfur trioxide.
- Show Video Lesson
Try the free Mathway calculator and
problem solver below to practice various math topics. Try the given examples, or type in your own
problem and check your answer with the step-by-step explanations.
We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.

A balanced chemical equation represents a chemical reaction as chemical formulas and numbers. Here is a collection of more than 10 balanced chemical equations. Use them as homework examples or to review the principles of balancing equations.
Balanced Equation Basics
- Elements are represented using their element symbols.
- The left side of the reaction lists the reactants, the right side lists the products, and the reaction arrow indicates the direction in which the reaction proceeds.
- In a balanced chemical equation, the same number and type of atoms are present on both sides of the reaction arrow.
- The number in front of a chemical formula is its coefficient and is the number of moles of that element or compound. If there is 1 mole of a substance, the number is omitted (e.g., write CO instead of 1 CO).
- Subscripts after an element symbol indicate the number of atoms of the element in a substance. If there is no subscript, it means there is one atom of that element.
- The total number of atoms in a compound is the subscript multiplied by the coefficient (e.g., 4H2O contains 4 x 2 = 8 atoms of hydrogen and 1 x 4 = 4 atoms of oxygen).
Balanced Chemical Equations
6 CO2 + 6 H2O → C6H12O6 + 6 O2 (balanced equation for photosynthesis)
2 AgI + Na2S → Ag2S + 2 NaI
Ba3N2 + 6 H2O → 3 Ba(OH)2 + 2 NH3
3 CaCl2 + 2 Na3PO4 → Ca3(PO4)2 + 6 NaCl
4 FeS + 7 O2 → 2 Fe2O3 + 4 SO2
PCl5 + 4 H2O → H3PO4 + 5 HCl
2 As + 6 NaOH → 2 Na3AsO3 + 3 H2
3 Hg(OH)2 + 2 H3PO4 → Hg3(PO4)2 + 6 H2O
12 HClO4 + P4O10 → 4 H3PO4 + 6 Cl2O7
8 CO + 17 H2 → C8H18 + 8 H2O
10 KClO3 + 3 P4 → 3 P4O10 + 10 KCl
SnO2 + 2 H2 → Sn + 2 H2O
3 KOH + H3PO4 → K3PO4 + 3 H2O
2 KNO3 + H2CO3 → K2CO3 + 2 HNO3
Na3PO4 + 3 HCl → 3 NaCl + H3PO4
TiCl4 + 2 H2O → TiO2 + 4 HCl
C2H6O + 3 O2 → 2 CO2 + 3 H2O
2 Fe + 6 HC2H3O2 → 2 Fe(C2H3O2)3 + 3 H2
4 NH3 + 5 O2 → 4 NO + 6 H2O
B2Br6 + 6 HNO3 → 2 B(NO3)3 + 6 HBr
4 NH4OH + KAl(SO4)2·12H2O → Al(OH)3 + 2 (NH4)2SO4 + KOH + 12 H2O
Balanced Chemical Equations as Word Equations
Sometimes you may be asked to say a balanced chemical equation as a word equation. To read an equation aloud, you need to know the chemical name of the substance. The coefficients are read as “X moles of”, the subscripts aren’t stated because they are implied in the chemical name, and the reaction arrow is read as “yields” or “forms”.
For example, the following equation:
4 NH3 + 5 O2 → 4 NO + 6 H2O
Is read as:
Four moles of ammonia plus five moles of oxygen yields four moles of nitric oxide plus six moles of water.
Check Your Work
When you write a balanced equation, you should check your work to make certain it’s balanced and that it is written in its most-reduced form.
- Count the number of atoms of each element on both sides of the reaction arrow. They should be the same.
- Make certain all elements are included. If an element appears on one side of the reaction, it must also appear on the other side.
- Check to see if you can factor out the coefficients. For example, if all coefficients can be divided by 2, the equation may be balanced, but it could be written as a simpler balanced equation. Ideally, the equation should list the smallest mole ratios of reactants and products.
References
- Brady, James E.; Senese, Frederick; Jespersen, Neil D. (2007). Chemistry: Matter and Its Changes. John Wiley & Sons. ISBN 9780470120941.
- Crosland, M.P. (1959). “The use of diagrams as chemical ‘equations’ in the lectures of William Cullen and Joseph Black”. Annals of Science. 15 (2): 75–90. doi:10.1080/00033795900200088
- Thorne, Lawrence R. (2010). “An Innovative Approach to Balancing Chemical-Reaction Equations: A Simplified Matrix-Inversion Technique for Determining the Matrix Null Space”. Chem. Educator. 15: 304–308.
Загрузить PDF
Загрузить PDF
Химическое уравнение — это символическое представление химической реакции. При этом вступающие в реакцию соединения (реагенты) пишутся в левой, а получившиеся вещества (продукты реакции) — в правой части уравнения. Между ними ставится стрелка слева направо, которая указывает направление реакции. Согласно закону сохранения массы, в ходе химической реакции не могут появиться новые атомы или исчезнуть старые, поэтому количество атомов в реагентах должно быть равно числу атомов в продуктах химической реакции. В данной статье описано, как приводить химические уравнения к балансу с помощью разных методов.[1]
-
1
Запишите химическое уравнение. В качестве примера рассмотрим следующую реакцию:
- C3H8 + O2 –> H2O + CO2
- Эта реакция описывает горение пропана (C3H8) в присутствии кислорода с образованием воды и диоксида углерода (углекислого газа).
-
2
Запишите количество атомов каждого элемента. Сделайте это для обеих частей уравнения. Обратите внимание на подстрочные индексы возле каждого элемента, чтобы определить общее количество атомов. Запишите символ каждого входящего в уравнение элемента и отметьте соответствующее количество атомов.[2]
- Например, в правой части рассматриваемого уравнения в результате сложения получаем 3 атома кислорода.
- В левой части имеем 3 атома углерода (C3), 8 атомов водорода (H8) и 2 атома кислорода (O2).
- В правой части имеем 1 атом углерода (C), 2 атома водорода (H2) и 3 атома кислорода (O + O2).
-
3
Оставьте водород и кислород на потом, так как они входят в состав нескольких соединений в левой и правой части. Водород и кислород входят в состав нескольких молекул, поэтому лучше сбалансировать их в последнюю очередь.[3]
- Прежде чем балансировать водород и кислород, придется еще раз пересчитать атомы, так как могут понадобиться дополнительные коэффициенты, чтобы сбалансировать другие элементы.
-
4
Начните с наименее часто встречающегося элемента. Если необходимо сбалансировать несколько элементов, выберите такой, который входит в состав одной молекулы реагентов и одной молекулы продуктов реакции. Таким образом, сначала следует сбалансировать углерод.[4]
-
5
Для баланса добавьте коэффициент перед единственным атомом углерода. Поставьте коэффициент перед единственным атомом углерода в правой части уравнения, чтобы сбалансировать его с 3 атомами углерода в левой части.[5]
- C3H8 + O2 –> H2O + 3CO2
- Коэффициент 3 перед углеродом в правой части уравнения указывает на то, что получается три атома углерода, которые соответствуют тремя атомам углерода, входящим в молекулу пропана в левой части.
- В химическом уравнении можно менять коэффициенты перед атомами и молекулами, однако подстрочные индексы должны оставаться неизменными.
-
6
После этого сбалансируйте атомы водорода. После того как вы уравняли количество атомов углерода в левой и правой части, несбалансированными остались водород и кислород. Левая часть уравнения содержит 8 атомов водорода, столько же их должно быть и справа. Добейтесь этого с помощью коэффициента.[6]
- C3H8 + O2 –> 4H2O + 3CO2
- Мы добавили коэффициент 4 в правой части, так как подстрочный индекс показывает, что у нас уже есть два атома водорода.
- Если умножить коэффициент 4 на подстрочный индекс 2, получится 8.
- В результате в правой части получается 10 атомов кислорода: 3×2=6 атомов в трех молекулах 3CO2 и еще четыре атома в четырех молекулах воды.
-
7
Сбалансируйте атомы кислорода. Не забудьте учесть коэффициенты, которые вы использовали для балансировки других атомов. Поскольку вы добавили коэффициенты перед молекулами в правой части уравнения, количество атомов кислорода изменилось. Теперь у вас 4 атома кислорода в молекулах воды и 6 атомов кислорода в молекулах диоксида углерода. Таким, образом, в правую часть входит 10 атомов кислорода.[7]
- Добавьте коэффициент 5 к молекуле кислорода в левой части уравнения. Теперь каждая часть содержит по 10 атомов кислорода.
- C3H8 + 5O2 –> 4H2O + 3CO2.
- Итак, обе части уравнения содержат одинаковое количество атомов углерода, водорода и кислорода. Уравнение сбалансировано.
Реклама
-
1
Запишите уравнение реакции. В качестве примера рассмотрим следующую химическую реакцию:
- PCl5 + H2O –> H3PO4 + HCl
-
2
Поставьте букву перед каждым соединением:
- aPCl5 + bH2O –> cH3PO4 + dHCl
-
3
Приравняйте количество атомов каждого элемента в левой и правой частях уравнения.[8]
- aPCl5 + bH2O –> cH3PO4 + dHCl
- Слева имеем 2b атомов водорода (по 2 в каждой молекуле H2O), в то время как справа 3c+d атомов водорода (по 3 в каждой молекуле H3PO4 и по 1 в каждой молекуле HCl). Поскольку левая и правая часть должны содержать одинаковое число атомов водорода, 2b должно быть равно 3c+d.
- Сделайте это для всех элементов:
- P: a=c
- Cl: 5a=d
- H: 2b=3c+d
-
4
Решите систему уравнений, чтобы найти численные значения коэффициентов. Система имеет несколько решений, так как переменных больше, чем уравнений. Необходимо найти такое решение, чтобы все коэффициенты имели вид минимально возможных целых чисел.[9]
- Чтобы быстро решить систему уравнений, присвоим численное значение одной из переменных. Предположим, a=1. Решим систему и найдем значения остальных переменных:
- Для P a = c, поэтому c = 1
- Для Cl 5a = d, поэтому d = 5
- Поскольку для H 2b = 3c + d, находим величину b:
- 2b = 3(1) + 5
- 2b = 3 + 5
- 2b = 8
- b=4
- Таким образом, имеем следующие коэффициенты:
- a = 1
- b = 4
- c = 1
- d = 5
Реклама
Советы
- Если вы испытываете трудности, для балансировки уравнений химических реакций можно использовать онлайн-калькулятор. Однако учтите, что таким калькулятором не разрешается пользоваться во время экзамена, поэтому не полагайтесь лишь на него.
- Помните, что иногда уравнение можно упростить! Если все коэффициенты делятся без остатка на целое число, упростите уравнение.
Реклама
Предупреждения
- Чтобы избавиться от дробных коэффициентов, умножьте все уравнение (его левую и правую часть) на знаменатель дроби.
- Никогда не используйте в качестве коэффициентов химического уравнения дроби — в химических реакциях не бывает половин молекул или атомов.
- В процессе балансировки для удобства можно использовать дроби, однако уравнение не сбалансировано до тех пор, пока в нем остаются дробные коэффициенты.
Реклама
Об этой статье
Эту страницу просматривали 53 441 раз.
Была ли эта статья полезной?
Presentation on theme: «Balancing Chemical Equations. Writing Word Equations Steps in writing equations: 1.word equation A.types of reactions B.predicting products 2.formula.»— Presentation transcript:
1
Balancing Chemical Equations
2
Writing Word Equations Steps in writing equations: 1.word equation A.types of reactions B.predicting products 2.formula equation (unbalanced) 3.balanced chemical equation 4.net ionic equation (for precipitation reaction)
3
Types of Chemical Reactions
4
1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion A + B → AB AB → A + B AB+ C → AC + B AB+ CD → AD + CB CH+ O 2 → CO 2 + H 2 O (N.B., in a double replacement, cations stay in front)
5
Examples of Chemical Reactions 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion 2H 2 O → 2H 2 + O 2 2Al + 3CuCl 2 → 2AlCl 3 + 3Cu AgNO 3 + NaCl → AgCl + NaNO 3 1C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O 2H 2 + O 2 → 2H 2 O (cellular respiration)
6
Types of Chemical Reactions 1. Synthesis 2. Decomposition 3. Single Replacement 4. Double Replacement 5. Combustion A + B → AB AB → A + B AB+ C → AC + B AB+ CD → AD + CB CH+ O 2 → CO 2 + H 2 O N.B. A.“replacement” or “displacement” B.in a double replacement, cations stay in front C.subtypes – e.g,. 2NaCl + 1F 2 2NaF + 2Na is a halogen s.r. reaction
7
Steps to completing a balanced chemical equation. Given words of reactants. 1.Determine the type of reaction. 2.Write the words for the products. (even when given the reactants formulas) 3.Translate the words into formulas. 4.Balance 5.Check
8
Ag + CuSO 4 1.Single replacement (cation replacement) 2.Ag + CuSO 4 copper + silver chloride (Silver is +1) 3.Ag + CuSO 4 Cu + AgCl 4.1Ag + 1CuSO 4 1Cu + 1AgCl
Of all the skills to know about in chemistry, balancing chemical equations is perhaps the most important to master. So many parts of chemistry depend on this vital skill, including stoichiometry, reaction analysis, and lab work. This comprehensive guide will show you the steps to balance even the most challenging reactions and will walk you through a series of examples, from simple to complex.
The Key to Balancing Chemical Equations
The ultimate goal for balancing chemical equations is to make both sides of the reaction, the reactants and the products, equal in the number of atoms per element. This stems from the universal law of the conservation of mass, which states that matter can neither be created nor destroyed. So, if we start with ten atoms of oxygen before a reaction, we need to end up with ten atoms of oxygen after a reaction. This means that chemical reactions do not change the actual building blocks of matter; rather, they just change the arrangement of the blocks. An easy way to understand this is to picture a house made of blocks. We can break the house apart and build an airplane, but the color and shape of the actual blocks do not change.
But how do we go about balancing these equations? We know that the number of atoms of each element needs to be the same on both sides of the equation, so it is just a matter of finding the correct coefficients (numbers in front of each molecule) to make that happen. It is best to start with the atom that shows up the least number of times on one side, and balance that first. Then, move on to the atom that shows up the second least number of times, and so on. In the end, make sure to count the number of atoms of each element on each side again, just to be sure.
Example of Balancing a Chemical Equation
Let’s illustrate this with an example by balancing this chemical equation:
P4O10 + H2O → H3PO4
First, let’s look at the element that appears least often. Notice that oxygen occurs twice on the left-hand side, so that is not a good element to start out with. We could either start with phosphorus or hydrogen, so let’s start with phosphorus. There are four atoms of phosphorus on the left-hand side, but only one on the right-hand side. So, we can put the coefficient of 4 on the molecule that has phosphorous on the right-hand side to balance them out.
P4O10 + H2O → 4 H3PO4
Now we can check hydrogen. We still want to avoid balancing oxygen, because it occurs in more than one molecule on the left-hand side. It is easiest to start with molecules that only appear once on each side. So, there are two molecules of hydrogen on the left-hand side and twelve on the right-hand side (notice that there are three per molecule of H3PO4, and we have four molecules). So, to balance those out, we have to put a six in front of H2O on the left.
P4O10 + 6 H2O → 4 H3PO4
At this point, we can check the oxygens to see if they balance. On the left, we have ten atoms of oxygen from P4O10 and six from H2O for a total of 16. On the right, we have 16 as well (four per molecule, with four molecules). So, oxygen is already balanced. This gives us the final balanced equation of
P4O10 + 6 H2O → 4 H3PO4
Try to balance these ten equations on your own, then check the answers below. They range in difficulty level, so don’t get discouraged if some of them seem too hard. Just remember to start with the element that shows up the least, and proceed from there. The best way to approach these problems is slowly and systematically. Looking at everything at once can easily get overwhelming. Good luck!
- CO2 + H2O → C6H12O6 + O2
- SiCl4 + H2O → H4SiO4 + HCl
- Al + HCl → AlCl3 + H2
- Na2CO3 + HCl → NaCl + H2O + CO2
- C7H6O2 + O2 → CO2 + H2O
- Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3
- Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3
- KClO3 → KClO4 + KCl
- Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4
- H2SO4 + HI → H2S + I2 + H2O
Complete Solutions:
1. CO2 + H2O → C6H12O6 + O2
The first step to balancing chemical equations is to focus on elements that only appear once on each side of the equation. Here, both carbon and hydrogen fit this requirement. So, we will start with carbon. There is only one atom of carbon on the left-hand side, but six on the right-hand side. So, we add a coefficient of six to the carbon-containing molecule on the left.
6CO2 + H2O → C6H12O6 + O2
Next, let’s look at hydrogen. There are two hydrogen atoms on the left and twelve on the right. So, we will add a coefficient of six on the hydrogen-containing molecule on the left.
6CO2 + 6H2O → C6H12O6 + O2
Now, it is time to check the oxygen. There are a total of 18 oxygen molecules on the left (6×2 + 6×1). On the right, there are eight oxygen molecules. Now, we have two options to even out the right-hand side: We can either multiply C6H12O6 or O2 by a coefficient. However, if we change C6H12O6, the coefficients for everything else on the left-hand side will also have to change, because we will be changing the number of carbon and hydrogen atoms. To prevent this, it usually helps to only change the molecule containing the fewest elements; in this case, the O2. So, we can add a coefficient of six to the O2 on the right. Our final answer will be:
6CO2 + 6H2O → C6H12O6 + 6O2
2. SiCl4 + H2O → H4SiO4 + HCl
The only element that occurs more than once on the same side of the equation here is hydrogen, so we can start with any other element. Let’s start by looking at silicon. Notice that there is only one atom of silicon on either side, so we do not need to add any coefficients yet. Next, let’s look at chlorine. There are four chlorine atoms on the left side and only one on the right. So, we will add a coefficient of four on the right.
SiCl4 + H2O → H4SiO4 + 4HCl
Next, let’s look at oxygen. Remember that we first want to analyze all the elements that only occur once on one side of the equation. There is only one oxygen atom on the left, but four on the right. So, we will add a coefficient of four on the left-hand side of the equation.
SiCl4 + 4H2O → H4SiO4 + 4HCl
We are almost done! Now, we just have to check the number of hydrogen atoms on each side. The left has eight and the right also has eight, so we are done. Our final answer is
SiCl4 + 4H2O → H4SiO4 + 4HCl
As always, make sure to double-check that the number of atoms of each element balances on each side before continuing.
3. Al + HCl → AlCl3 + H2
This problem is a bit tricky, so be careful. Whenever a single atom is alone on either side of the equation, it is easiest to start with that element. So, we will start by counting the aluminum atoms on both sides. There is one on the left and one on the right, so we do not need to add any coefficients yet. Next, let’s look at hydrogen. There is also one on the left, but two on the right. So, we will add a coefficient of two on the left.
Al + 2HCl → AlCl3 + H2
Next, we will look at chlorine. There are now two on the left, but three on the right. Now, this is not as straightforward as just adding a coefficient to one side. We need the number of chlorine atoms to be equal on both sides, so we need to get two and three to be equal. We can accomplish this by finding the lowest common multiple. In this case, we can multiply two by three and three by two to get the lowest common multiple of six. So, we will multiply 2HCl by three and AlCl3 by two:
Al + 6HCl → 2AlCl3 + H2
We have looked at all the elements, so it is easy to say that we are done. However, always make sure to double-check. In this case, because we added a coefficient to the aluminum-containing molecule on the right-hand side, aluminum is no longer balanced. There is one on the left but two on the right. So, we will add one more coefficient.
2Al + 6HCl → 2AlCl3 + H2
We are not quite done yet. Looking over the equation one final time, we see that hydrogen has also been unbalanced. There are six on the left but two on the right. So, with one final adjustment, we get our final answer:
2Al + 6HCl → 2AlCl3 + 3H2
4. Na2CO3 + HCl → NaCl + H2O + CO2
Hopefully, by this point, balancing equations is becoming easier and you are getting the hang of it. Looking at sodium, we see that it occurs twice on the left, but once on the right. So, we can add our first coefficient to the NaCl on the right.
Na2CO3 + HCl → 2NaCl + H2O + CO2
Next, let’s look at carbon. There is one on the left and one on the right, so there are no coefficients to add. Since oxygen occurs in more than one place on the left, we will save it for last. Instead, look at hydrogen. There is one on the left and two on the right, so we will add a coefficient to the left.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
Then, looking at chlorine, we see that it is already balanced with two on each side. Now we can go back to look at oxygen. There are three on the left and three on the right, so our final answer is
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
5. C7H6O2 + O2 → CO2 + H2O
We can start balancing this equation by looking at either carbon or hydrogen. Looking at carbon, we see that there are seven atoms on the left and only one on the right. So, we can add a coefficient of seven on the right.
C7H6O2 + O2 → 7CO2 + H2O
Then, for hydrogen, there are six atoms on the left and two on the right. So, we will add a coefficient of three on the right.
C7H6O2 + O2→ 7CO2 + 3H2O
Now, for oxygen, things will get a little tricky. Oxygen occurs in every molecule in the equation, so we have to be very careful when balancing it. There are four atoms of oxygen on the left and 17 on the right. There is no obvious way to balance these numbers, so we must use a little trick: fractions. Now, when balancing chemical equations, we cannot include fractions as it is not proper form, but it sometimes helps to use them to solve the problem. Also, try to avoid over-manipulating organic molecules. You can easily identify organic molecules, otherwise known as CHO molecules, because they are made up of only carbon, hydrogen, and oxygen. We don’t like to work with these molecules, because they are rather complex. Also, larger molecules tend to be more stable than smaller molecules, and less likely to react in large quantities.
So, to balance out the four and seventeen, we can multiply the O2 on the left by 7.5. That will give us
C7H6O2 + 7.5O2 → 7CO2 + 3H2O
Remember, fractions (and decimals) are not allowed in formal balanced equations, so multiply everything by two to get integer values. Our final answer is now
2C7H6O2 + 15O2 → 14CO2 + 6H2O
6. Fe2(SO4)3 + KOH → K2SO4 + Fe(OH)3-
We can start by balancing the iron on both sides. The left has two while the right only has one. So, we will add a coefficient of two to the right.
Fe2(SO4)3 + KOH → K2SO4 + 2Fe(OH)3-
Then, we can look at sulfur. There are three on the left, but only one on the right. So, we will add a coefficient of three to the right-hand side.
Fe2(SO4)3 + KOH → 3K2SO4 + 2Fe(OH)3-
We are almost done. All that is left is to balance the potassium. There is one atom on the left and six on the right, so we can balance these by adding a coefficient of six. Our final answer, then, is
Fe2(SO4)3 + 6KOH → 3K2SO4 + 2Fe(OH)3-
7. Ca3(PO4)2 + SiO2 → P4O10 + CaSiO3
Looking at calcium, we see that there are three on the left and one on the right, so we can add a coefficient of three on the right to balance them out.
Ca3(PO4)2 + SiO2 → P4O10 + 3CaSiO3
Then, for phosphorus, we see that there are two on the left and four on the right. To balance these, add a coefficient of two on the left.
2Ca3(PO4)2 + SiO2 → P4O10 + 3CaSiO3
Notice that by doing so, we changed the number of calcium atoms on the left. Every time you add a coefficient, double check to see if the step affects any elements you have already balanced. In this case, the number of calcium atoms on the left has increased to six while it is still three on the right, so we can change the coefficient on the right to reflect this change.
2Ca3(PO4)2 + SiO2 → P4O10 + 6CaSiO3
Since oxygen occurs in every molecule in the equation, we will skip it for now. Focusing on silicon, we see that there is one on the left, but six on the right, so we can add a coefficient to the left.
2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3
Now, we will check the number of oxygen atoms on each side. The left has 28 atoms and the right also has 28. So, after checking that all the other atoms are the same on both sides as well, we get a final answer of
2Ca3(PO4)2 + 6SiO2 → P4O10 + 6CaSiO3
8. KClO3 → KClO4 + KCl
This problem is particularly tricky because every atom, except oxygen, occurs in every molecule in the equation. So, since oxygen appears the least number of times, we will start there. There are three on the left and four on the right. To balance these, we find the lowest common multiple; in this case, 12. By adding a coefficient of four on the left and three on the right, we can balance the oxygens.
4KClO3 → 3KClO4 + KCl
Now, we can check potassium and chlorine. There are four potassium molecules on the left and four on the right, so they are balanced. Chlorine is also balanced, with four on each side, so we are finished, with a final answer of
4KClO3 → 3KClO4 + KCl
9. Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4
We can start here by balancing the aluminum atoms on both sides. The left has two molecules while the right only has one, so we will add a coefficient of two on the right.
Al2(SO4)3 + Ca(OH)2 → 2Al(OH)3 + CaSO4
Now, we can check sulfur. There are three on the left and only one on the right, so adding a coefficient of three will balance these.
Al2(SO4)3 + Ca(OH)2 → 2Al(OH)3 + 3CaSO4
Moving right along to calcium, there is only one on the left but three on the right, so we should add a coefficient of three.
Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
Double-checking all the atoms, we see that all the elements are balanced, so our final equation is
Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
10. H2SO4 + HI → H2S + I2 + H2O
Since hydrogen occurs more than once on the left, we will temporarily skip it and move to sulfur. There is one atom on the left and one on the right, so there is nothing to balance yet. Looking at oxygen, there are four on the left and one on the right, so we can add a coefficient of four to balance them.
H2SO4 + HI → H2S + I2 + 4H2O
There is only one iodine on the left and two on the right, so a simple coefficient change can balance those.
H2SO4 + 2HI → H2S + I2 + 4H2O
Now, we can look at the most challenging element: hydrogen. On the left, there are four and on the right, there are ten. So, we know we have to change the coefficient of either H2SO4 or HI. We want to change something that will require the least amount of tweaking afterwards, so we will change the coefficient of HI. To get the left-hand side to have ten atoms of hydrogen, we need HI to have eight atoms of hydrogen, since H2SO4 already has two. So, we will change the coefficient from 2 to 8.
H2SO4 + 8HI → H2S + I2 + 4H2O
However, this also changes the balance for iodine. There are now eight on the left, but only two on the right. To fix this, we will add a coefficient of 4 on the right. After checking that everything else balances out as well, we get a final answer of
H2SO4 + 8HI → H2S + 4I2 + 4H2O
As with most skills, practice makes perfect when balancing chemical equations. Keep working hard and try to do as many problems as you can to help you hone your balancing skills.
Writing Equations
Nothing created — nothing destroyed
- New substances are made during chemical reactions
- However, the same atoms are always present before and after reaction
- They have just joined up in different ways
- Atoms cannot be created or destroyed, so if they exist in the reactants then they absolutely must be in the products!
- Because of this the total mass of reactants is always equal to the total mass of products
- This idea is known as the Law of Conservation of Mass
Conservation of Mass
- The Law of Conservation of Mass enables us to balance chemical equations, since no atoms can be lost or created
- You should be able to:
- Write word equations for reactions outlined in these notes
- Write formulae and balanced chemical equations for the reactions in these notes
Word Equations
- These show the reactants and products of a chemical reaction using their full chemical names
- The reactants are those substances on the left-hand side of the arrow and can be thought of as the chemical ingredients of the reaction
- They react with each other and form new substances
- The products are the new substances which are on the right-hand side of the arrow
- The arrow (which is spoken as “goes to” or “produces”) implies the conversion of reactants into products
- Reaction conditions or the name of a catalyst (a substance added to make a reaction go faster) can be written above the arrow
- An example is the reaction of sodium hydroxide (a base) and hydrochloric acid produces sodium chloride (common table salt) and water:
sodium hydroxide + hydrochloric acid ⟶ sodium chloride + water
Representing reactions as equations
- Chemical equations use the chemical symbols of each reactant and product
- When balancing equations, there has to be the same number of atoms of each element on either side of the equation in accordance with the Law of Conservation of Mass
- A symbol equation uses the formulae of the reactants and products to show what happens in a chemical reaction
- A symbol equation must be balanced to give the correct ratio of reactants and products:
S + O2 → SO2
- This equation shows that one atom of sulfur (S) reacts with one molecule of oxygen (O2) to make one molecule of sulfur dioxide (SO2)
- The following non-metals must be written as molecules: H2, N2, O2, F2, Cl2, Br2 and I2
- To balance an equation you work across the equation from left to right, checking one element after another
- If there is a group of atoms, for example a nitrate group (NO3–) that has not changed from one side to the other, then count the whole group as one entity rather than counting the individual atoms
- Examples of chemical equations:
- Acid-base neutralisation reaction:
NaOH (aq) + HCl (aq) ⟶ NaCl (aq) + H2O (l)
-
- Redox reaction:
2Fe2O3 (aq) + 3C (s) ⟶ 4Fe (s) + 3CO2 (g)
- In each equation there are equal numbers of each atom on either side of the reaction arrow so the equations are balanced
- Don’t forget to add state symbols when writing balanced equations:
Balancing Equations
The best approach is to practice lot of examples of balancing equations
- By trial and error change the coefficients (multipliers) in front of the formulae, one by one checking the result on the other side
- Balance elements that appear on their own, last in the process
Worked Example
Example 1
Balance the following equation:
aluminium + copper(II)oxide ⟶ aluminium oxide + copper
Unbalanced symbol equation:
Al + CuO ⟶ Al2O3 + Cu
Answer
Worked Example
Example 2
Balance the following equation:
magnesium oxide + nitric acid ⟶ magnesium nitrate + water
Unbalanced symbol equation:
MgO + HNO3 ⟶ Mg(NO3)2 + H2O
Answer
Exam Tip
Chemical equations do not contain an equals sign between the left and right-hand sides but are written with an arrow instead. The arrow means that the reactants have reacted together and formed the product(s).
Writing ionic equations
- In aqueous solutions ionic compounds dissociate into their ions, meaning they separate into the component ions that formed them, e.g. hydrochloric acid and potassium hydroxide dissociate as follows:
HCl (aq) → H+ (aq) + Cl—(aq)
KOH (aq) → K+ (aq) + OH— (aq)
- It is important that you can recognise common ionic compounds and their constituent ions
- These include:
- Acids such as HCl and H2SO4
- Group I and Group II hydroxides e.g. sodium hydroxide
- Soluble salts e.g. potassium sulfate, sodium chloride
- Follow the example below to write ionic equations
Worked Example
Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide.
Answer:
Step 1: Write out the full balanced equation:
2KI (aq) + Cl2 (aq) → 2KCl (aq) + I2 (aq)
Step 2: Identify the ionic substances and write down the ions separately
2K+ (aq) + 2I— (aq) + Cl2 (aq) → 2K+ (aq) + 2Cl— (aq) + I2 (aq)
Step 3: Rewrite the equation eliminating the ions which appear on both sides of the equation (spectator ions ) which in this case are the K+ ions:
2I— (aq) + Cl2 (aq) → 2Cl— (aq) + I2 (aq)
Core Concepts
In this tutorial you will learn the process of balancing chemical equations as well as the components that make up a chemical equation.
Introduction to Balancing Chemical Equations
Balancing a chemical equation means making sure that the same number of atoms of each element are present on both sides of the equation. This is important because the law of conservation of mass states that matter cannot be created or destroyed, so the number of atoms of each element must remain the same during a chemical reaction. To balance a chemical equation, you need to carefully examine the reactants (the substances that are combined) and the products (the substances that are formed) and make sure that they have the same number of atoms of each element. This is usually done by adding coefficients (numbers in front of the chemical formulas) to the reactants and products to balance the equation.
For example, the equation for the combustion of methane (CH4) can be balanced by adding a coefficient of 2 to the oxygen on the right side of the equation:
CH4 + 2 O2 –> CO2 + 2 H2O
In this equation, the coefficients ensure that there are the same number of carbon, hydrogen, and oxygen atoms on both sides of the arrow, which indicates that the equation is balanced.
What is a Chemical Equation?
A chemical equation represents a chemical reaction using symbols and numbers. Chemical equations show the relative molar quantities and identities of different reactants and products. Balancing chemical equations can help you determine the accurate ratio of compounds involved in a reaction!
Fundamentals of a Chemical Equation
1. Basic parts of a chemical equation
There are 3 basic parts to a chemical equation: reactants, products, and the “yields” arrow.
| Reactants | Yields | Products | |
| Definition | Reactants are compounds or elements that are needed in the reaction and that undergo chemical change during the reaction. | In reactions, “yield” means to “produce” or “form” and is usually represented by an arrow. When reactants undergo a chemical change, they yield products. | Products are the end results, or compounds produced, in a chemical reaction. |
| Example | CH4 (g) + 2O2 (g) | → | CO2 (g) + 2H2O (g) |
The reactants and products are listed as multiple molecules added together, and this is the case for any chemical equation. The yields arrow, however, can come in a few different styles:
- Forward arrow (→) shows a general reaction.
- Equilibrium arrow (⇌) shows a reversible process.
- Resonance arrow (↔) shows that two or more species are resonance structures of each other.
- Theoretical arrow (⇢) shows a theoretical process.
- Retrosynthetic arrow (⇒) shows potentially ways to get from reactants to products.
2. Numbers in chemical equations
There are two types of numbers in chemical equations: subscripts and coefficients. Subscripts represent how many atoms of each element are present in a molecule. Coefficients represent how many molecules of a specific chemical compound are present in the reaction. Only coefficients of molecules can be changed in balancing equations.
Molecules in which the subscripts of atoms of the same elements are different (for example H2O vs. H2O2) have different chemical compositions, meaning they are not the same compound. For this reason, when balancing chemical equations, subscripts of molecules cannot be changed. The same molecules having different coefficients (for example 2H2O and 5H2O) are the same compounds, present in different amounts.
In a molecule 3NO2, the coefficient is 3 (there are 3 molecules of NO2), and the subscript of oxygen is 2 (there are 2 oxygen atoms in 1 molecule of NO2). In total, there are 6 oxygen atoms (3 molecules of NO2 and 2 oxygen atoms per 1 molecule of NO2.)
The coefficients of each molecule or compound represent the relative quantity of the element in the reaction in moles or numbers of molecules. Because they are relative quantities, the numbers can also be interpreted as ratios; in the example above, the ratio would be 1 CH4 : 2O2 : 1 CO2 : 2H2O. As long as the ratios are constant, the equation can be correctly rewritten with any proportional coefficients (such as 2:4:2:4, 3:6:3:6, etc.), though it is common practice to use the smallest whole number coefficients.
3. The Law of Conservation of Mass
The law of conservation of mass states that matter (or atoms) are neither created nor destroyed in chemical reactions. This means that there must be equal amounts of each compound before and after a chemical reaction takes place. The law applies to balancing chemical equations because there must be the same relative amounts of each compound on both sides of the equation.
Here’s an example of a reaction in which methane burns in air to produce carbon dioxide and water vapor:
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
As you can see, both sides of the equation each have 1 carbon atom, 4 hydrogen atoms, and 4 oxygen atoms. Thus the equation is balanced.
Steps to Writing & How to Balance Chemical Equations
How do you balance a chemical equation? Let’s look at the steps.
- Identifying the names of the reactants and products. It can be helpful to write a word equation to list out all the compounds in the reaction. In a word equation, the reactants and products are represented by their names, meaning they are written in word form, instead of as a molecular formula.
- It is important to remember that word equations only show the names of compounds, and not the quantities. This means that word equations do not show the full picture of the reaction.
- Using the word equation, write a formula equation by rewriting all of the compounds into their chemical formula.
- Formula equations are not balanced. They only show the compounds in their chemical formula.
- Balance the formula equation using the law of conservation of mass to write the chemical equation.
- Start by counting the atoms of elements that only appear once on each side of the equation and balance those first. Balance atoms that appear multiple times on each side of the equation last.
- Usually, balance any hydrogen or oxygen atoms last.
- After balancing all the atoms, you have a balanced formula equation, or chemical equation.
Balancing Chemical Equations – Example
Let’s look at how to balance chemical equations.
Balance the reaction: methane burns in air and combines with oxygen to produce carbon dioxide and water vapor
1. Write the Word Equation
Write the reaction as a word equation: methane + oxygen → carbon dioxide + water
2. Rewrite the Word Equation as a Formula Equation
Rewrite as formula equation: CH4 (g) + O2 (g) → CO2 (g) + H2O (g)
3. Balance the formula equation using the law of conservation of mass.
- Count the atoms of elements that appear once in the equation. In this example, carbon and hydrogen atoms appear once, and oxygen appears twice on the right side.
- Balance hydrogen and oxygen atoms last. Since oxygen appears twice, you would balance oxygen last. In the example, you would start by balancing carbon, then hydrogen, and oxygen last.
- There is one carbon atom on the left side of the equation (CH4), and one on the right side (CO2). Therefore, carbon is already balanced. Both compounds containing carbon (CH4 and CO2) should have a coefficient of 1, because they must exist equally to have an equal number of carbon atoms.
- There are 4 hydrogen atoms on the left side of the equation (CH4), and 2 on the right side (H2O). You will need 2 more hydrogen atoms on the right side to balance the hydrogen atoms. Multiplying the coefficient of H2O by 2 gives 2H2O, which has 4 hydrogen atoms.
- It is important not to try to change H2O to H4O in an effort to balance the hydrogen atoms. Doing so changes the chemical identity, and H4O is not water. Multiplying the entire compound (H2O) changes the number of water molecules in the reaction, without altering its chemical identity.
- The formula equation is now: 1CH4 (g) + 1O2 (g) → 1CO2 (g) + 2H2O (g)
- There are now 2 oxygen atoms on the left side (O2) and 4 on the right side (2H2O and CO2). Multiplying the coefficient of O2 by 2 gives 2O2, which has 4 oxygen atoms.
- After all the atoms are balanced, you have a chemical equation: CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O (g)
4. Check the balanced equation by counting all the atoms.
| Left Side | Right Side |
| Carbon atoms – 1 (CH4) | Carbon atoms – 1 (CO2) |
| Hydrogen atoms – 4 (CH4) | Hydrogen atoms – 4 (2H2O) |
| Oxygen atoms – 4 (2O2) | Oxygen atoms – 4 (2 from CO2, 2 from 2H2O) |
Further Reading
- How to Write Net Ionic Equations
- Balance Redox Reactions
- Types of Chemical Reactions
- Limiting Reactant