| Atom | |
|---|---|
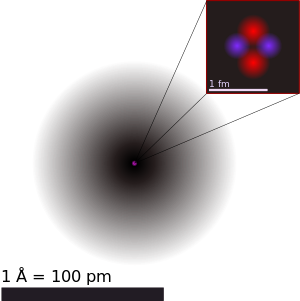
An illustration of the helium atom, depicting the nucleus (pink) and the electron cloud distribution (black). The nucleus (upper right) in helium-4 is in reality spherically symmetric and closely resembles the electron cloud, although for more complicated nuclei this is not always the case. The black bar is one angstrom (10−10 m or 100 pm). |
|
| Classification | |
| Smallest recognized division of a chemical element | |
| Properties | |
| Mass range | 1.67×10−27 to 4.52×10−25 kg |
| Electric charge | zero (neutral), or ion charge |
| Diameter range | 62 pm (He) to 520 pm (Cs) (data page) |
| Components | Electrons and a compact nucleus of protons and neutrons |
An atom is a particle that consists of a nucleus of protons and neutrons surrounded by a cloud of electrons. The atom is the basic particle of the chemical elements, and the chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. The number of neutrons defines the isotope of the element.
Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. This is smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. Atoms are so small that accurately predicting their behavior using classical physics is not possible due to quantum effects.
More than 99.94% of an atom’s mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus splits and leaves behind different elements. This is a form of nuclear decay.
Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to attach and detach is responsible for most of the physical changes observed in nature. Chemistry is the discipline that studies these changes.
History of atomic theory
In philosophy
The basic idea that matter is made up of tiny indivisible particles is an old idea that appeared in many ancient cultures. The word atom is derived from the ancient Greek word atomos,[a] which means «uncuttable». This ancient idea was based in philosophical reasoning rather than scientific reasoning. Modern atomic theory is not based on these old concepts.[1][2] In the early 19th century, the scientist John Dalton noticed that chemical elements seemed to combine with each other by discrete units of weight, and he decided to use the word «atom» to refer to these units, as he thought these were the fundamental units of matter.[3] About a century later it was discovered that Dalton’s atoms are not actually indivisible, but the term stuck.
Dalton’s law of multiple proportions
Atoms and molecules as depicted in John Dalton’s A New System of Chemical Philosophy vol. 1 (1808)
In the early 1800s, the English chemist John Dalton compiled experimental data gathered by himself and other scientists and discovered a pattern now known as the «law of multiple proportions». He noticed that in chemical compounds which contain a particular chemical element, the content of that element in these compounds will differ in weight by ratios of small whole numbers. This pattern suggested that each chemical element combines with other elements by a basic unit of weight, and Dalton decided to call these units «atoms».
For example, there are two types of tin oxide: one is a grey powder that is 88.1% tin and 11.9% oxygen, and the other is a white powder that is 78.7% tin and 21.3% oxygen. Adjusting these figures, in the grey powder there is about 13.5 g of oxygen for every 100 g of tin, and in the white powder there is about 27 g of oxygen for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. Dalton concluded that in these oxides, for every tin atom there are one or two oxygen atoms respectively (SnO and SnO2).[4][5]
Dalton also analyzed iron oxides. There is one type of iron oxide that is a black powder which is 78.1% iron and 21.9% oxygen; and there is another iron oxide that is a red powder which is 70.4% iron and 29.6% oxygen. Adjusting these figures, in the black powder there is about 28 g of oxygen for every 100 g of iron, and in the red powder there is about 42 g of oxygen for every 100 g of iron. 28 and 42 form a ratio of 2:3. Dalton concluded that in these oxides, for every two atoms of iron, there are two or three atoms of oxygen respectively (Fe2O2 and Fe2O3).[b][6][7]
As a final example: nitrous oxide is 63.3% nitrogen and 36.7% oxygen, nitric oxide is 44.05% nitrogen and 55.95% oxygen, and nitrogen dioxide is 29.5% nitrogen and 70.5% oxygen. Adjusting these figures, in nitrous oxide there is 80 g of oxygen for every 140 g of nitrogen, in nitric oxide there is about 160 g of oxygen for every 140 g of nitrogen, and in nitrogen dioxide there is 320 g of oxygen for every 140 g of nitrogen. 80, 160, and 320 form a ratio of 1:2:4. The respective formulas for these oxides are N2O, NO, and NO2.[8][9]
Isomerism
Scientists discovered some substances have the exact same chemical content but different properties. For instance, in 1827, Friedrich Wöhler discovered that silver fulminate and silver cyanate are both 107 parts silver, 12 parts carbon, 14 parts nitrogen, and 12 parts oxygen (we now know their formulas as both AgCNO). In 1830 Jöns Jacob Berzelius introduced the term isomerism to describe the phenomenon. In 1860, Louis Pasteur hypothesized that the molecules of isomers might have the same composition but different arrangements of their atoms.[10]
In 1874, Jacobus Henricus van ‘t Hoff proposed that the carbon atom bonds to other atoms in a tetrahedral arrangement. Working from this, he explained the structures of organic molecules in such a way that he could predict how many isomers a compound could have. Consider, for example, pentane (C5H12). In van ‘t Hoff’s way of modelling molecules, there are three possible configurations for pentane, and there really are three different isomers of pentane in nature.[11][12]
Brownian motion
In 1827, the British botanist Robert Brown observed that dust particles inside pollen grains floating in water constantly jiggled about for no apparent reason. In 1905, Albert Einstein theorized that this Brownian motion was caused by the water molecules continuously knocking the grains about, and developed a mathematical model to describe it.[13] This model was validated experimentally in 1908 by French physicist Jean Perrin, who used Einstein’s equation to calculate the number of atoms in a mole and the size of atoms.[14]
Discovery of the electron
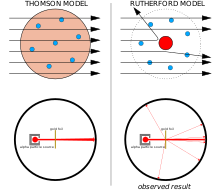
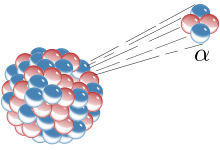
The Geiger–Marsden experiment:
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
In 1897, J. J. Thomson discovered that cathode rays are not electromagnetic waves but made of particles because they can be deflected by electrical and magnetic fields. He measured these particles to be 1,800 times lighter than hydrogen (the lightest atom). Thomson concluded that these particles came from the atoms within the cathode — they were subatomic particles. He called these new particles corpuscles but they were later renamed electrons. Thomson also showed that electrons were identical to particles given off by photoelectric and radioactive materials.[15] It was quickly recognized that electrons are the particles that carry electric currents in metal wires.[16] Thomson concluded that these electrons emerged from the very atoms of the cathode in his instruments, which meant that atoms are not indivisible as Dalton thought.
Discovery of the nucleus
J. J. Thomson thought that the negatively-charged electrons were distributed throughout the atom in a sea of positive charge that was distributed across the whole volume of the atom.[17] This model is sometimes known as the plum pudding model.
Ernest Rutherford and his colleagues Hans Geiger and Ernest Marsden came to doubt the Thomson model after they encountered difficulties when they tried to build an instrument to measure the charge-to-mass ratio of alpha particles (these are positively-charged particles emitted by certain radioactive substances such as radium). The alpha particles were being scattered by the air in the detection chamber, which made the measurements unreliable. Thomson had encountered a similar problem in his work on cathode rays, which he solved by creating a near-perfect vacuum in his instruments. Rutherford didn’t think he’d run into this same problem because alpha particles are much heavier than electrons. According to Thomson’s model of the atom, the positive charge in the atom is not concentrated enough to produce an electric field strong enough to deflect an alpha particle, and the electrons are so lightweight they should be pushed aside effortlessly by the much heavier alpha particles. Yet there was scattering, so Rutherford and his colleagues decided to investigate this scattering carefully.[18]
Between 1908 and 1913, Rutherford and his colleagues performed a series of experiments in which they bombarded thin foils of metal with alpha particles. They spotted alpha particles being deflected by angles greater than 90°. To explain this, Rutherford proposed that the positive charge of the atom is not distributed throughout the atom’s volume as Thomson believed, but is concentrated in a tiny nucleus at the center. Only such an intense concentration of charge could produce an electric field strong enough to deflect the alpha particles as observed.[18]
Discovery of isotopes
While experimenting with the products of radioactive decay, in 1913 radiochemist Frederick Soddy discovered that there appeared to be more than one type of atom at each position on the periodic table.[19] The term isotope was coined by Margaret Todd as a suitable name for different atoms that belong to the same element. J. J. Thomson created a technique for isotope separation through his work on ionized gases, which subsequently led to the discovery of stable isotopes.[20]
Bohr model
The Bohr model of the atom, with an electron making instantaneous «quantum leaps» from one orbit to another with gain or loss of energy. This model of electrons in orbits is obsolete.
In 1913, the physicist Niels Bohr proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon.[21] This quantization was used to explain why the electrons’ orbits are stable (given that normally, charges in acceleration, including circular motion, lose kinetic energy which is emitted as electromagnetic radiation, see synchrotron radiation) and why elements absorb and emit electromagnetic radiation in discrete spectra.[22]
Later in the same year Henry Moseley provided additional experimental evidence in favor of Niels Bohr’s theory. These results refined Ernest Rutherford’s and Antonius van den Broek’s model, which proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. Until these experiments, atomic number was not known to be a physical and experimental quantity. That it is equal to the atomic nuclear charge remains the accepted atomic model today.[23]
Chemical bonds between atoms were explained by Gilbert Newton Lewis in 1916, as the interactions between their constituent electrons.[24] As the chemical properties of the elements were known to largely repeat themselves according to the periodic law,[25] in 1919 the American chemist Irving Langmuir suggested that this could be explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of electron shells about the nucleus.[26]
The Bohr model of the atom was the first complete physical model of the atom. It described the overall structure of the atom, how atoms bond to each other, and predicted the spectral lines of hydrogen. Bohr’s model was not perfect and was soon superseded by the more accurate Schrödinger model, but it was sufficient to evaporate any remaining doubts that matter is composed of atoms. For chemists, the idea of the atom had been a useful heuristic tool, but physicists had doubts as to whether matter really is made up of atoms as nobody had yet developed a complete physical model of the atom.
The Schrödinger model
The Stern–Gerlach experiment of 1922 provided further evidence of the quantum nature of atomic properties. When a beam of silver atoms was passed through a specially shaped magnetic field, the beam was split in a way correlated with the direction of an atom’s angular momentum, or spin. As this spin direction is initially random, the beam would be expected to deflect in a random direction. Instead, the beam was split into two directional components, corresponding to the atomic spin being oriented up or down with respect to the magnetic field.[27]
In 1925, Werner Heisenberg published the first consistent mathematical formulation of quantum mechanics (matrix mechanics).[23] One year earlier, Louis de Broglie had proposed the de Broglie hypothesis: that all particles behave like waves to some extent,[28] and in 1926 Erwin Schrödinger used this idea to develop the Schrödinger equation, a mathematical model of the atom (wave mechanics) that described the electrons as three-dimensional waveforms rather than point particles.[29]
A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at a given point in time. This became known as the uncertainty principle, formulated by Werner Heisenberg in 1927.[23] In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa.[30]
This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and spectral patterns of atoms larger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described atomic orbital zones around the nucleus where a given electron is most likely to be observed.[31][32]
Discovery of the neutron
The development of the mass spectrometer allowed the mass of atoms to be measured with increased accuracy. The device uses a magnet to bend the trajectory of a beam of ions, and the amount of deflection is determined by the ratio of an atom’s mass to its charge. The chemist Francis William Aston used this instrument to show that isotopes had different masses. The atomic mass of these isotopes varied by integer amounts, called the whole number rule.[33] The explanation for these different isotopes awaited the discovery of the neutron, an uncharged particle with a mass similar to the proton, by the physicist James Chadwick in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.[34]
Fission, high-energy physics and condensed matter
In 1938, the German chemist Otto Hahn, a student of Rutherford, directed neutrons onto uranium atoms expecting to get transuranium elements. Instead, his chemical experiments showed barium as a product.[35][36] A year later, Lise Meitner and her nephew Otto Frisch verified that Hahn’s result were the first experimental nuclear fission.[37][38] In 1944, Hahn received the Nobel Prize in Chemistry. Despite Hahn’s efforts, the contributions of Meitner and Frisch were not recognized.[39]
In the 1950s, the development of improved particle accelerators and particle detectors allowed scientists to study the impacts of atoms moving at high energies.[40] Neutrons and protons were found to be hadrons, or composites of smaller particles called quarks. The standard model of particle physics was developed that so far has successfully explained the properties of the nucleus in terms of these sub-atomic particles and the forces that govern their interactions.[41]
Structure
Subatomic particles
Though the word atom originally denoted a particle that cannot be cut into smaller particles, in modern scientific usage the atom is composed of various subatomic particles. The constituent particles of an atom are the electron, the proton and the neutron.
The electron is by far the least massive of these particles at 9.11×10−31 kg, with a negative electrical charge and a size that is too small to be measured using available techniques.[42] It was the lightest particle with a positive rest mass measured, until the discovery of neutrino mass. Under ordinary conditions, electrons are bound to the positively charged nucleus by the attraction created from opposite electric charges. If an atom has more or fewer electrons than its atomic number, then it becomes respectively negatively or positively charged as a whole; a charged atom is called an ion. Electrons have been known since the late 19th century, mostly thanks to J.J. Thomson; see history of subatomic physics for details.
Protons have a positive charge and a mass 1,836 times that of the electron, at 1.6726×10−27 kg. The number of protons in an atom is called its atomic number. Ernest Rutherford (1919) observed that nitrogen under alpha-particle bombardment ejects what appeared to be hydrogen nuclei. By 1920 he had accepted that the hydrogen nucleus is a distinct particle within the atom and named it proton.
Neutrons have no electrical charge and have a free mass of 1,839 times the mass of the electron, or 1.6749×10−27 kg.[43][44] Neutrons are the heaviest of the three constituent particles, but their mass can be reduced by the nuclear binding energy. Neutrons and protons (collectively known as nucleons) have comparable dimensions—on the order of 2.5×10−15 m—although the ‘surface’ of these particles is not sharply defined.[45] The neutron was discovered in 1932 by the English physicist James Chadwick.
In the Standard Model of physics, electrons are truly elementary particles with no internal structure, whereas protons and neutrons are composite particles composed of elementary particles called quarks. There are two types of quarks in atoms, each having a fractional electric charge. Protons are composed of two up quarks (each with charge +2/3) and one down quark (with a charge of −1/3). Neutrons consist of one up quark and two down quarks. This distinction accounts for the difference in mass and charge between the two particles.[46][47]
The quarks are held together by the strong interaction (or strong force), which is mediated by gluons. The protons and neutrons, in turn, are held to each other in the nucleus by the nuclear force, which is a residuum of the strong force that has somewhat different range-properties (see the article on the nuclear force for more). The gluon is a member of the family of gauge bosons, which are elementary particles that mediate physical forces.[46][47]
Nucleus
The binding energy needed for a nucleon to escape the nucleus, for various isotopes
All the bound protons and neutrons in an atom make up a tiny atomic nucleus, and are collectively called nucleons. The radius of a nucleus is approximately equal to ![{displaystyle 1.07{sqrt[{3}]{A}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a74a6ca6998768195969eef75ca046e8431c29d3)

Atoms of the same element have the same number of protons, called the atomic number. Within a single element, the number of neutrons may vary, determining the isotope of that element. The total number of protons and neutrons determine the nuclide. The number of neutrons relative to the protons determines the stability of the nucleus, with certain isotopes undergoing radioactive decay.[50]
The proton, the electron, and the neutron are classified as fermions. Fermions obey the Pauli exclusion principle which prohibits identical fermions, such as multiple protons, from occupying the same quantum state at the same time. Thus, every proton in the nucleus must occupy a quantum state different from all other protons, and the same applies to all neutrons of the nucleus and to all electrons of the electron cloud.[51]
A nucleus that has a different number of protons than neutrons can potentially drop to a lower energy state through a radioactive decay that causes the number of protons and neutrons to more closely match. As a result, atoms with matching numbers of protons and neutrons are more stable against decay, but with increasing atomic number, the mutual repulsion of the protons requires an increasing proportion of neutrons to maintain the stability of the nucleus.[51]
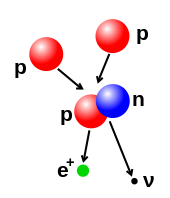
Illustration of a nuclear fusion process that forms a deuterium nucleus, consisting of a proton and a neutron, from two protons. A positron (e+)—an antimatter electron—is emitted along with an electron neutrino.
The number of protons and neutrons in the atomic nucleus can be modified, although this can require very high energies because of the strong force. Nuclear fusion occurs when multiple atomic particles join to form a heavier nucleus, such as through the energetic collision of two nuclei. For example, at the core of the Sun protons require energies of 3 to 10 keV to overcome their mutual repulsion—the coulomb barrier—and fuse together into a single nucleus.[52] Nuclear fission is the opposite process, causing a nucleus to split into two smaller nuclei—usually through radioactive decay. The nucleus can also be modified through bombardment by high energy subatomic particles or photons. If this modifies the number of protons in a nucleus, the atom changes to a different chemical element.[53][54]
If the mass of the nucleus following a fusion reaction is less than the sum of the masses of the separate particles, then the difference between these two values can be emitted as a type of usable energy (such as a gamma ray, or the kinetic energy of a beta particle), as described by Albert Einstein’s mass-energy equivalence formula, 


The fusion of two nuclei that create larger nuclei with lower atomic numbers than iron and nickel—a total nucleon number of about 60—is usually an exothermic process that releases more energy than is required to bring them together.[56] It is this energy-releasing process that makes nuclear fusion in stars a self-sustaining reaction. For heavier nuclei, the binding energy per nucleon in the nucleus begins to decrease. That means fusion processes producing nuclei that have atomic numbers higher than about 26, and atomic masses higher than about 60, is an endothermic process. These more massive nuclei can not undergo an energy-producing fusion reaction that can sustain the hydrostatic equilibrium of a star.[51]
Electron cloud
A potential well, showing, according to classical mechanics, the minimum energy V(x) needed to reach each position x. Classically, a particle with energy E is constrained to a range of positions between x1 and x2.
The electrons in an atom are attracted to the protons in the nucleus by the electromagnetic force. This force binds the electrons inside an electrostatic potential well surrounding the smaller nucleus, which means that an external source of energy is needed for the electron to escape. The closer an electron is to the nucleus, the greater the attractive force. Hence electrons bound near the center of the potential well require more energy to escape than those at greater separations.
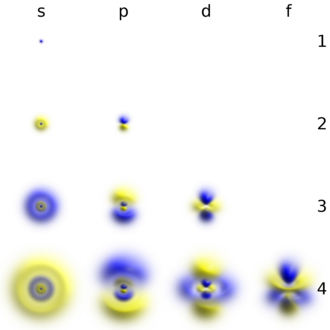
Electrons, like other particles, have properties of both a particle and a wave. The electron cloud is a region inside the potential well where each electron forms a type of three-dimensional standing wave—a wave form that does not move relative to the nucleus. This behavior is defined by an atomic orbital, a mathematical function that characterises the probability that an electron appears to be at a particular location when its position is measured.[57] Only a discrete (or quantized) set of these orbitals exist around the nucleus, as other possible wave patterns rapidly decay into a more stable form.[58] Orbitals can have one or more ring or node structures, and differ from each other in size, shape and orientation.[59]
3D views of some hydrogen-like atomic orbitals showing probability density and phase (g orbitals and higher are not shown)
Each atomic orbital corresponds to a particular energy level of the electron. The electron can change its state to a higher energy level by absorbing a photon with sufficient energy to boost it into the new quantum state. Likewise, through spontaneous emission, an electron in a higher energy state can drop to a lower energy state while radiating the excess energy as a photon. These characteristic energy values, defined by the differences in the energies of the quantum states, are responsible for atomic spectral lines.[58]
The amount of energy needed to remove or add an electron—the electron binding energy—is far less than the binding energy of nucleons. For example, it requires only 13.6 eV to strip a ground-state electron from a hydrogen atom,[60] compared to 2.23 million eV for splitting a deuterium nucleus.[61] Atoms are electrically neutral if they have an equal number of protons and electrons. Atoms that have either a deficit or a surplus of electrons are called ions. Electrons that are farthest from the nucleus may be transferred to other nearby atoms or shared between atoms. By this mechanism, atoms are able to bond into molecules and other types of chemical compounds like ionic and covalent network crystals.[62]
Properties
Nuclear properties
By definition, any two atoms with an identical number of protons in their nuclei belong to the same chemical element. Atoms with equal numbers of protons but a different number of neutrons are different isotopes of the same element. For example, all hydrogen atoms admit exactly one proton, but isotopes exist with no neutrons (hydrogen-1, by far the most common form,[63] also called protium), one neutron (deuterium), two neutrons (tritium) and more than two neutrons. The known elements form a set of atomic numbers, from the single-proton element hydrogen up to the 118-proton element oganesson.[64] All known isotopes of elements with atomic numbers greater than 82 are radioactive, although the radioactivity of element 83 (bismuth) is so slight as to be practically negligible.[65][66]
About 339 nuclides occur naturally on Earth,[67] of which 251 (about 74%) have not been observed to decay, and are referred to as «stable isotopes». Only 90 nuclides are stable theoretically, while another 161 (bringing the total to 251) have not been observed to decay, even though in theory it is energetically possible. These are also formally classified as «stable». An additional 35 radioactive nuclides have half-lives longer than 100 million years, and are long-lived enough to have been present since the birth of the Solar System. This collection of 286 nuclides are known as primordial nuclides. Finally, an additional 53 short-lived nuclides are known to occur naturally, as daughter products of primordial nuclide decay (such as radium from uranium), or as products of natural energetic processes on Earth, such as cosmic ray bombardment (for example, carbon-14).[68][note 1]
For 80 of the chemical elements, at least one stable isotope exists. As a rule, there is only a handful of stable isotopes for each of these elements, the average being 3.1 stable isotopes per element. Twenty-six «monoisotopic elements» have only a single stable isotope, while the largest number of stable isotopes observed for any element is ten, for the element tin. Elements 43, 61, and all elements numbered 83 or higher have no stable isotopes.[69]: 1–12
Stability of isotopes is affected by the ratio of protons to neutrons, and also by the presence of certain «magic numbers» of neutrons or protons that represent closed and filled quantum shells. These quantum shells correspond to a set of energy levels within the shell model of the nucleus; filled shells, such as the filled shell of 50 protons for tin, confers unusual stability on the nuclide. Of the 251 known stable nuclides, only four have both an odd number of protons and odd number of neutrons: hydrogen-2 (deuterium), lithium-6, boron-10, and nitrogen-14. (Tantalum-180m is odd-odd and observationally stable, but is predicted to decay with a very long half-life.) Also, only four naturally occurring, radioactive odd-odd nuclides have a half-life over a billion years: potassium-40, vanadium-50, lanthanum-138, and lutetium-176. Most odd-odd nuclei are highly unstable with respect to beta decay, because the decay products are even-even, and are therefore more strongly bound, due to nuclear pairing effects.[70]
Mass
The large majority of an atom’s mass comes from the protons and neutrons that make it up. The total number of these particles (called «nucleons») in a given atom is called the mass number. It is a positive integer and dimensionless (instead of having dimension of mass), because it expresses a count. An example of use of a mass number is «carbon-12,» which has 12 nucleons (six protons and six neutrons).
The actual mass of an atom at rest is often expressed in daltons (Da), also called the unified atomic mass unit (u). This unit is defined as a twelfth of the mass of a free neutral atom of carbon-12, which is approximately 1.66×10−27 kg.[71] Hydrogen-1 (the lightest isotope of hydrogen which is also the nuclide with the lowest mass) has an atomic weight of 1.007825 Da.[72] The value of this number is called the atomic mass. A given atom has an atomic mass approximately equal (within 1%) to its mass number times the atomic mass unit (for example the mass of a nitrogen-14 is roughly 14 Da), but this number will not be exactly an integer except (by definition) in the case of carbon-12.[73] The heaviest stable atom is lead-208,[65] with a mass of 207.9766521 Da.[74]
As even the most massive atoms are far too light to work with directly, chemists instead use the unit of moles. One mole of atoms of any element always has the same number of atoms (about 6.022×1023). This number was chosen so that if an element has an atomic mass of 1 u, a mole of atoms of that element has a mass close to one gram. Because of the definition of the unified atomic mass unit, each carbon-12 atom has an atomic mass of exactly 12 Da, and so a mole of carbon-12 atoms weighs exactly 0.012 kg.[71]
Shape and size
Atoms lack a well-defined outer boundary, so their dimensions are usually described in terms of an atomic radius. This is a measure of the distance out to which the electron cloud extends from the nucleus.[75] This assumes the atom to exhibit a spherical shape, which is only obeyed for atoms in vacuum or free space. Atomic radii may be derived from the distances between two nuclei when the two atoms are joined in a chemical bond. The radius varies with the location of an atom on the atomic chart, the type of chemical bond, the number of neighboring atoms (coordination number) and a quantum mechanical property known as spin.[76] On the periodic table of the elements, atom size tends to increase when moving down columns, but decrease when moving across rows (left to right).[77] Consequently, the smallest atom is helium with a radius of 32 pm, while one of the largest is caesium at 225 pm.[78]
When subjected to external forces, like electrical fields, the shape of an atom may deviate from spherical symmetry. The deformation depends on the field magnitude and the orbital type of outer shell electrons, as shown by group-theoretical considerations. Aspherical deviations might be elicited for instance in crystals, where large crystal-electrical fields may occur at low-symmetry lattice sites.[79][80] Significant ellipsoidal deformations have been shown to occur for sulfur ions[81] and chalcogen ions[82] in pyrite-type compounds.
Atomic dimensions are thousands of times smaller than the wavelengths of light (400–700 nm) so they cannot be viewed using an optical microscope, although individual atoms can be observed using a scanning tunneling microscope. To visualize the minuteness of the atom, consider that a typical human hair is about 1 million carbon atoms in width.[83] A single drop of water contains about 2 sextillion (2×1021) atoms of oxygen, and twice the number of hydrogen atoms.[84] A single carat diamond with a mass of 2×10−4 kg contains about 10 sextillion (1022) atoms of carbon.[note 2] If an apple were magnified to the size of the Earth, then the atoms in the apple would be approximately the size of the original apple.[85]
Radioactive decay
This diagram shows the half-life (T1⁄2) of various isotopes with Z protons and N neutrons.
Every element has one or more isotopes that have unstable nuclei that are subject to radioactive decay, causing the nucleus to emit particles or electromagnetic radiation. Radioactivity can occur when the radius of a nucleus is large compared with the radius of the strong force, which only acts over distances on the order of 1 fm.[86]
The most common forms of radioactive decay are:[87][88]
- Alpha decay: this process is caused when the nucleus emits an alpha particle, which is a helium nucleus consisting of two protons and two neutrons. The result of the emission is a new element with a lower atomic number.
- Beta decay (and electron capture): these processes are regulated by the weak force, and result from a transformation of a neutron into a proton, or a proton into a neutron. The neutron to proton transition is accompanied by the emission of an electron and an antineutrino, while proton to neutron transition (except in electron capture) causes the emission of a positron and a neutrino. The electron or positron emissions are called beta particles. Beta decay either increases or decreases the atomic number of the nucleus by one. Electron capture is more common than positron emission, because it requires less energy. In this type of decay, an electron is absorbed by the nucleus, rather than a positron emitted from the nucleus. A neutrino is still emitted in this process, and a proton changes to a neutron.
- Gamma decay: this process results from a change in the energy level of the nucleus to a lower state, resulting in the emission of electromagnetic radiation. The excited state of a nucleus which results in gamma emission usually occurs following the emission of an alpha or a beta particle. Thus, gamma decay usually follows alpha or beta decay.
Other more rare types of radioactive decay include ejection of neutrons or protons or clusters of nucleons from a nucleus, or more than one beta particle. An analog of gamma emission which allows excited nuclei to lose energy in a different way, is internal conversion—a process that produces high-speed electrons that are not beta rays, followed by production of high-energy photons that are not gamma rays. A few large nuclei explode into two or more charged fragments of varying masses plus several neutrons, in a decay called spontaneous nuclear fission.
Each radioactive isotope has a characteristic decay time period—the half-life—that is determined by the amount of time needed for half of a sample to decay. This is an exponential decay process that steadily decreases the proportion of the remaining isotope by 50% every half-life. Hence after two half-lives have passed only 25% of the isotope is present, and so forth.[86]
Magnetic moment
Elementary particles possess an intrinsic quantum mechanical property known as spin. This is analogous to the angular momentum of an object that is spinning around its center of mass, although strictly speaking these particles are believed to be point-like and cannot be said to be rotating. Spin is measured in units of the reduced Planck constant (ħ), with electrons, protons and neutrons all having spin 1⁄2 ħ, or «spin-1⁄2«. In an atom, electrons in motion around the nucleus possess orbital angular momentum in addition to their spin, while the nucleus itself possesses angular momentum due to its nuclear spin.[89]
The magnetic field produced by an atom—its magnetic moment—is determined by these various forms of angular momentum, just as a rotating charged object classically produces a magnetic field, but the most dominant contribution comes from electron spin. Due to the nature of electrons to obey the Pauli exclusion principle, in which no two electrons may be found in the same quantum state, bound electrons pair up with each other, with one member of each pair in a spin up state and the other in the opposite, spin down state. Thus these spins cancel each other out, reducing the total magnetic dipole moment to zero in some atoms with even number of electrons.[90]
In ferromagnetic elements such as iron, cobalt and nickel, an odd number of electrons leads to an unpaired electron and a net overall magnetic moment. The orbitals of neighboring atoms overlap and a lower energy state is achieved when the spins of unpaired electrons are aligned with each other, a spontaneous process known as an exchange interaction. When the magnetic moments of ferromagnetic atoms are lined up, the material can produce a measurable macroscopic field. Paramagnetic materials have atoms with magnetic moments that line up in random directions when no magnetic field is present, but the magnetic moments of the individual atoms line up in the presence of a field.[90][91]
The nucleus of an atom will have no spin when it has even numbers of both neutrons and protons, but for other cases of odd numbers, the nucleus may have a spin. Normally nuclei with spin are aligned in random directions because of thermal equilibrium, but for certain elements (such as xenon-129) it is possible to polarize a significant proportion of the nuclear spin states so that they are aligned in the same direction—a condition called hyperpolarization. This has important applications in magnetic resonance imaging.[92][93]
Energy levels
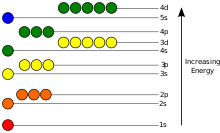
These electron’s energy levels (not to scale) are sufficient for ground states of atoms up to cadmium (5s2 4d10) inclusively. Do not forget that even the top of the diagram is lower than an unbound electron state.
The potential energy of an electron in an atom is negative relative to when the distance from the nucleus goes to infinity; its dependence on the electron’s position reaches the minimum inside the nucleus, roughly in inverse proportion to the distance. In the quantum-mechanical model, a bound electron can occupy only a set of states centered on the nucleus, and each state corresponds to a specific energy level; see time-independent Schrödinger equation for a theoretical explanation. An energy level can be measured by the amount of energy needed to unbind the electron from the atom, and is usually given in units of electronvolts (eV). The lowest energy state of a bound electron is called the ground state, i.e. stationary state, while an electron transition to a higher level results in an excited state.[94] The electron’s energy increases along with n because the (average) distance to the nucleus increases. Dependence of the energy on ℓ is caused not by the electrostatic potential of the nucleus, but by interaction between electrons.
For an electron to transition between two different states, e.g. ground state to first excited state, it must absorb or emit a photon at an energy matching the difference in the potential energy of those levels, according to the Niels Bohr model, what can be precisely calculated by the Schrödinger equation.
Electrons jump between orbitals in a particle-like fashion. For example, if a single photon strikes the electrons, only a single electron changes states in response to the photon; see Electron properties.
The energy of an emitted photon is proportional to its frequency, so these specific energy levels appear as distinct bands in the electromagnetic spectrum.[95] Each element has a characteristic spectrum that can depend on the nuclear charge, subshells filled by electrons, the electromagnetic interactions between the electrons and other factors.[96]
An example of absorption lines in a spectrum
When a continuous spectrum of energy is passed through a gas or plasma, some of the photons are absorbed by atoms, causing electrons to change their energy level. Those excited electrons that remain bound to their atom spontaneously emit this energy as a photon, traveling in a random direction, and so drop back to lower energy levels. Thus the atoms behave like a filter that forms a series of dark absorption bands in the energy output. (An observer viewing the atoms from a view that does not include the continuous spectrum in the background, instead sees a series of emission lines from the photons emitted by the atoms.) Spectroscopic measurements of the strength and width of atomic spectral lines allow the composition and physical properties of a substance to be determined.[97]
Close examination of the spectral lines reveals that some display a fine structure splitting. This occurs because of spin–orbit coupling, which is an interaction between the spin and motion of the outermost electron.[98] When an atom is in an external magnetic field, spectral lines become split into three or more components; a phenomenon called the Zeeman effect. This is caused by the interaction of the magnetic field with the magnetic moment of the atom and its electrons. Some atoms can have multiple electron configurations with the same energy level, which thus appear as a single spectral line. The interaction of the magnetic field with the atom shifts these electron configurations to slightly different energy levels, resulting in multiple spectral lines.[99] The presence of an external electric field can cause a comparable splitting and shifting of spectral lines by modifying the electron energy levels, a phenomenon called the Stark effect.[100]
If a bound electron is in an excited state, an interacting photon with the proper energy can cause stimulated emission of a photon with a matching energy level. For this to occur, the electron must drop to a lower energy state that has an energy difference matching the energy of the interacting photon. The emitted photon and the interacting photon then move off in parallel and with matching phases. That is, the wave patterns of the two photons are synchronized. This physical property is used to make lasers, which can emit a coherent beam of light energy in a narrow frequency band.[101]
Valence and bonding behavior
Valency is the combining power of an element. It is determined by the number of bonds it can form to other atoms or groups.[102] The outermost electron shell of an atom in its uncombined state is known as the valence shell, and the electrons in
that shell are called valence electrons. The number of valence electrons determines the bonding
behavior with other atoms. Atoms tend to chemically react with each other in a manner that fills (or empties) their outer valence shells.[103] For example, a transfer of a single electron between atoms is a useful approximation for bonds that form between atoms with one-electron more than a filled shell, and others that are one-electron short of a full shell, such as occurs in the compound sodium chloride and other chemical ionic salts. Many elements display multiple valences, or tendencies to share differing numbers of electrons in different compounds. Thus, chemical bonding between these elements takes many forms of electron-sharing that are more than simple electron transfers. Examples include the element carbon and the organic compounds.[104]
The chemical elements are often displayed in a periodic table that is laid out to display recurring chemical properties, and elements with the same number of valence electrons form a group that is aligned in the same column of the table. (The horizontal rows correspond to the filling of a quantum shell of electrons.) The elements at the far right of the table have their outer shell completely filled with electrons, which results in chemically inert elements known as the noble gases.[105][106]
States
Quantities of atoms are found in different states of matter that depend on the physical conditions, such as temperature and pressure. By varying the conditions, materials can transition between solids, liquids, gases and plasmas.[107] Within a state, a material can also exist in different allotropes. An example of this is solid carbon, which can exist as graphite or diamond.[108] Gaseous allotropes exist as well, such as dioxygen and ozone.
At temperatures close to absolute zero, atoms can form a Bose–Einstein condensate, at which point quantum mechanical effects, which are normally only observed at the atomic scale, become apparent on a macroscopic scale.[109][110] This super-cooled collection of atoms
then behaves as a single super atom, which may allow fundamental checks of quantum mechanical behavior.[111]
Identification
While atoms are too small to be seen, devices such as the scanning tunneling microscope (STM) enable their visualization at the surfaces of solids. The microscope uses the quantum tunneling phenomenon, which allows particles to pass through a barrier that would be insurmountable in the classical perspective. Electrons tunnel through the vacuum between two biased electrodes, providing a tunneling current that is exponentially dependent on their separation. One electrode is a sharp tip ideally ending with a single atom. At each point of the scan of the surface the tip’s height is adjusted so as to keep the tunneling current at a set value. How much the tip moves to and away from the surface is interpreted as the height profile. For low bias, the microscope images the averaged electron orbitals across closely packed energy levels—the local density of the electronic states near the Fermi level.[112][113] Because of the distances involved, both electrodes need to be extremely stable; only then periodicities can be observed that correspond to individual atoms. The method alone is not chemically specific, and cannot identify the atomic species present at the surface.
Atoms can be easily identified by their mass. If an atom is ionized by removing one of its electrons, its trajectory when it passes through a magnetic field will bend. The radius by which the trajectory of a moving ion is turned by the magnetic field is determined by the mass of the atom. The mass spectrometer uses this principle to measure the mass-to-charge ratio of ions. If a sample contains multiple isotopes, the mass spectrometer can determine the proportion of each isotope in the sample by measuring the intensity of the different beams of ions. Techniques to vaporize atoms include inductively coupled plasma atomic emission spectroscopy and inductively coupled plasma mass spectrometry, both of which use a plasma to vaporize samples for analysis.[114]
The atom-probe tomograph has sub-nanometer resolution in 3-D and can chemically identify individual atoms using time-of-flight mass spectrometry.[115]
Electron emission techniques such as X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES), which measure the binding energies of the core electrons, are used to identify the atomic species present in a sample in a non-destructive way. With proper focusing both can be made area-specific. Another such method is electron energy loss spectroscopy (EELS), which measures the energy loss of an electron beam within a transmission electron microscope when it interacts with a portion of a sample.
Spectra of excited states can be used to analyze the atomic composition of distant stars. Specific light wavelengths contained in the observed light from stars can be separated out and related to the quantized transitions in free gas atoms. These colors can be replicated using a gas-discharge lamp containing the same element.[116] Helium was discovered in this way in the spectrum of the Sun 23 years before it was found on Earth.[117]
Origin and current state
Baryonic matter forms about 4% of the total energy density of the observable universe, with an average density of about 0.25 particles/m3 (mostly protons and electrons).[118] Within a galaxy such as the Milky Way, particles have a much higher concentration, with the density of matter in the interstellar medium (ISM) ranging from 105 to 109 atoms/m3.[119] The Sun is believed to be inside the Local Bubble, so the density in the solar neighborhood is only about 103 atoms/m3.[120] Stars form from dense clouds in the ISM, and the evolutionary processes of stars result in the steady enrichment of the ISM with elements more massive than hydrogen and helium.
Up to 95% of the Milky Way’s baryonic matter are concentrated inside stars, where conditions are unfavorable for atomic matter. The total baryonic mass is about 10% of the mass of the galaxy;[121] the remainder of the mass is an unknown dark matter.[122] High temperature inside stars makes most «atoms» fully ionized, that is, separates all electrons from the nuclei. In stellar remnants—with exception of their surface layers—an immense pressure make electron shells impossible.
Formation
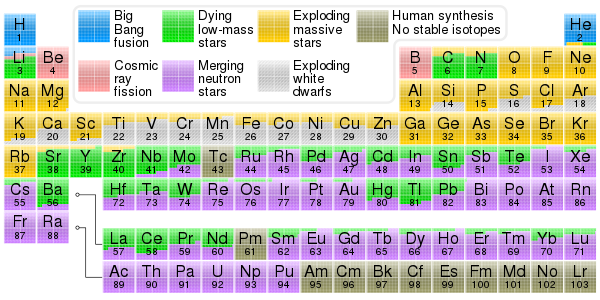
Periodic table showing the origin of each element. Elements from carbon up to sulfur may be made in small stars by the alpha process. Elements beyond iron are made in large stars with slow neutron capture (s-process). Elements heavier than iron may be made in neutron star mergers or supernovae after the r-process.
Electrons are thought to exist in the Universe since early stages of the Big Bang. Atomic nuclei forms in nucleosynthesis reactions. In about three minutes Big Bang nucleosynthesis produced most of the helium, lithium, and deuterium in the Universe, and perhaps some of the beryllium and boron.[123][124][125]
Ubiquitousness and stability of atoms relies on their binding energy, which means that an atom has a lower energy than an unbound system of the nucleus and electrons. Where the temperature is much higher than ionization potential, the matter exists in the form of plasma—a gas of positively charged ions (possibly, bare nuclei) and electrons. When the temperature drops below the ionization potential, atoms become statistically favorable. Atoms (complete with bound electrons) became to dominate over charged particles 380,000 years after the Big Bang—an epoch called recombination, when the expanding Universe cooled enough to allow electrons to become attached to nuclei.[126]
Since the Big Bang, which produced no carbon or heavier elements, atomic nuclei have been combined in stars through the process of nuclear fusion to produce more of the element helium, and (via the triple alpha process) the sequence of elements from carbon up to iron;[127] see stellar nucleosynthesis for details.
Isotopes such as lithium-6, as well as some beryllium and boron are generated in space through cosmic ray spallation.[128] This occurs when a high-energy proton strikes an atomic nucleus, causing large numbers of nucleons to be ejected.
Elements heavier than iron were produced in supernovae and colliding neutron stars through the r-process, and in AGB stars through the s-process, both of which involve the capture of neutrons by atomic nuclei.[129] Elements such as lead formed largely through the radioactive decay of heavier elements.[130]
Earth
Most of the atoms that make up the Earth and its inhabitants were present in their current form in the nebula that collapsed out of a molecular cloud to form the Solar System. The rest are the result of radioactive decay, and their relative proportion can be used to determine the age of the Earth through radiometric dating.[131][132] Most of the helium in the crust of the Earth (about 99% of the helium from gas wells, as shown by its lower abundance of helium-3) is a product of alpha decay.[133]
There are a few trace atoms on Earth that were not present at the beginning (i.e., not «primordial»), nor are results of radioactive decay. Carbon-14 is continuously generated by cosmic rays in the atmosphere.[134] Some atoms on Earth have been artificially generated either deliberately or as by-products of nuclear reactors or explosions.[135][136] Of the transuranic elements—those with atomic numbers greater than 92—only plutonium and neptunium occur naturally on Earth.[137][138] Transuranic elements have radioactive lifetimes shorter than the current age of the Earth[139] and thus identifiable quantities of these elements have long since decayed, with the exception of traces of plutonium-244 possibly deposited by cosmic dust.[131] Natural deposits of plutonium and neptunium are produced by neutron capture in uranium ore.[140]
The Earth contains approximately 1.33×1050 atoms.[141] Although small numbers of independent atoms of noble gases exist, such as argon, neon, and helium, 99% of the atmosphere is bound in the form of molecules, including carbon dioxide and diatomic oxygen and nitrogen. At the surface of the Earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. Atoms can also combine to create materials that do not consist of discrete molecules, including crystals and liquid or solid metals.[142][143] This atomic matter forms networked arrangements that lack the particular type of small-scale interrupted order associated with molecular matter.[144]
Rare and theoretical forms
Superheavy elements
All nuclides with atomic numbers higher than 82 (lead) are known to be radioactive. No nuclide with an atomic number exceeding 92 (uranium) exists on Earth as a primordial nuclide, and heavier elements generally have shorter half-lives. Nevertheless, an «island of stability» encompassing relatively long-lived isotopes of superheavy elements[145] with atomic numbers 110 to 114 might exist.[146] Predictions for the half-life of the most stable nuclide on the island range from a few minutes to millions of years.[147] In any case, superheavy elements (with Z > 104) would not exist due to increasing Coulomb repulsion (which results in spontaneous fission with increasingly short half-lives) in the absence of any stabilizing effects.[148]
Exotic matter
Each particle of matter has a corresponding antimatter particle with the opposite electrical charge. Thus, the positron is a positively charged antielectron and the antiproton is a negatively charged equivalent of a proton. When a matter and corresponding antimatter particle meet, they annihilate each other. Because of this, along with an imbalance between the number of matter and antimatter particles, the latter are rare in the universe. The first causes of this imbalance are not yet fully understood, although theories of baryogenesis may offer an explanation. As a result, no antimatter atoms have been discovered in nature.[149][150] In 1996, the antimatter counterpart of the hydrogen atom (antihydrogen) was synthesized at the CERN laboratory in Geneva.[151][152]
Other exotic atoms have been created by replacing one of the protons, neutrons or electrons with other particles that have the same charge. For example, an electron can be replaced by a more massive muon, forming a muonic atom. These types of atoms can be used to test fundamental predictions of physics.[153][154][155]
See also
- History of quantum mechanics
- Infinite divisibility
- Outline of chemistry
- Motion
- Timeline of atomic and subatomic physics
- Nuclear model
- Radioactive isotope
Notes
- ^ For more recent updates see Brookhaven National Laboratory’s Interactive Chart of Nuclides ] Archived 25 July 2020 at the Wayback Machine.
- ^ A carat is 200 milligrams. By definition, carbon-12 has 0.012 kg per mole. The Avogadro constant defines 6×1023 atoms per mole.
- ^ a combination of the negative term «a-» and «τομή,» the term for «cut»
- ^ Iron(II) oxide’s formula is written here as «Fe2O2» rather than the more conventional «FeO» because this better illustrates the explanation.
References
- ^ Pullman, Bernard (1998). The Atom in the History of Human Thought. Oxford, England: Oxford University Press. pp. 31–33. ISBN 978-0-19-515040-7. Archived from the original on 5 February 2021. Retrieved 25 October 2020.
- ^ Melsen (1952). From Atomos to Atom, pp. 18–19
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 198: «Dalton reaffirmed
that atoms are indivisible and indestructible and are the ultimate constituents of matter.» - ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, p. 36
- ^ Melsen (1952). From Atomos to Atom, p. 137
- ^ Dalton (1817). A New System of Chemical Philosophy vol. 2, pp. 28
- ^ Millington (1906). John Dalton, p. 113
- ^ Dalton (1808). A New System of Chemical Philosophy vol. 1, pp. 316–319
- ^ Holbrow et al. (2010). Modern Introductory Physics, pp. 65-66
- ^ Pullman (1998). The Atom in the History of Human Thought, p. 230
- ^ Melsen (1952). From Atomos to Atom, pp. 147-148
- ^ Henry Enfield Roscoe, Carl Schorlemmer (1895). A Treatise on Chemistry, Volume 3, Part 1, pp. 121-122
- ^ Einstein, A. (1905). «Über die von der molekularkinetischen Theorie der Wärme geforderte Bewegung von in ruhenden Flüssigkeiten suspendierten Teilchen» (PDF). Annalen der Physik. 322 (8): 549–560. Bibcode:1905AnP…322..549E. doi:10.1002/andp.19053220806. hdl:10915/2785.
- ^ «The Nobel Prize in Physics 1926». NobelPrize.org. Retrieved 8 February 2023.
- ^ Thomson, J.J. (August 1901). «On bodies smaller than atoms». The Popular Science Monthly: 323–335. Archived from the original on 1 December 2016. Retrieved 21 June 2009.
- ^ «The Mechanism Of Conduction In Metals» Archived 25 October 2012 at the Wayback Machine, Think Quest.
- ^ Navarro (2012). A History of the Electron, p. 94
- ^ a b Heilbron (2003). Ernest Rutherford and the Explosion of Atoms, pp. 64-68
- ^ «Frederick Soddy, The Nobel Prize in Chemistry 1921». Nobel Foundation. Archived from the original on 9 April 2008. Retrieved 18 January 2008.
- ^ Thomson, Joseph John (1913). «Rays of positive electricity». Proceedings of the Royal Society. A. 89 (607): 1–20. Bibcode:1913RSPSA..89….1T. doi:10.1098/rspa.1913.0057. Archived from the original on 4 November 2016.
- ^ Stern, David P. (16 May 2005). «The Atomic Nucleus and Bohr’s Early Model of the Atom». NASA/Goddard Space Flight Center. Archived from the original on 20 August 2007.
- ^ Bohr, Niels (11 December 1922). «Niels Bohr, The Nobel Prize in Physics 1922, Nobel Lecture». Nobel Foundation. Archived from the original on 15 April 2008.
- ^ a b c Pais, Abraham (1986). Inward Bound: Of Matter and Forces in the Physical World. New York: Oxford University Press. pp. 228–230. ISBN 978-0-19-851971-3.
- ^ Lewis, Gilbert N. (1916). «The Atom and the Molecule». Journal of the American Chemical Society. 38 (4): 762–786. doi:10.1021/ja02261a002. S2CID 95865413. Archived (PDF) from the original on 25 August 2019.
- ^ Scerri, Eric R. (2007). The periodic table: its story and its significance. Oxford University Press US. pp. 205–226. ISBN 978-0-19-530573-9.
- ^ Langmuir, Irving (1919). «The Arrangement of Electrons in Atoms and Molecules». Journal of the American Chemical Society. 41 (6): 868–934. doi:10.1021/ja02227a002. Archived from the original on 21 June 2019.
- ^ Scully, Marlan O.; Lamb, Willis E.; Barut, Asim (1987). «On the theory of the Stern-Gerlach apparatus». Foundations of Physics. 17 (6): 575–583. Bibcode:1987FoPh…17..575S. doi:10.1007/BF01882788. S2CID 122529426.
- ^ McEvoy, J. P.; Zarate, Oscar (2004). Introducing Quantum Theory. Totem Books. pp. 110–114. ISBN 978-1-84046-577-8.
- ^ Kozłowski, Miroslaw (2019). «The Schrödinger equation A History».
- ^ Chad Orzel (16 September 2014). «What is the Heisenberg Uncertainty Principle?». TED-Ed. Archived from the original on 13 September 2015 – via YouTube.
- ^ Brown, Kevin (2007). «The Hydrogen Atom». MathPages. Archived from the original on 5 September 2012.
- ^ Harrison, David M. (2000). «The Development of Quantum Mechanics». University of Toronto. Archived from the original on 25 December 2007.
- ^ Aston, Francis W. (1920). «The constitution of atmospheric neon». Philosophical Magazine. 39 (6): 449–455. doi:10.1080/14786440408636058. Archived from the original on 27 April 2021. Retrieved 25 October 2020.
- ^ Chadwick, James (12 December 1935). «Nobel Lecture: The Neutron and Its Properties». Nobel Foundation. Archived from the original on 12 October 2007.
- ^ Bowden, Mary Ellen (1997). «Otto Hahn, Lise Meitner, and Fritz Strassmann». Chemical achievers : the human face of the chemical sciences. Philadelphia, PA: Chemical Heritage Foundation. pp. 76–80, 125. ISBN 978-0-941901-12-3.
- ^ «Otto Hahn, Lise Meitner, and Fritz Strassmann». Science History Institute. June 2016. Archived from the original on 21 March 2018.
- ^ Meitner, Lise; Frisch, Otto Robert (1939). «Disintegration of uranium by neutrons: a new type of nuclear reaction». Nature. 143 (3615): 239–240. Bibcode:1939Natur.143..239M. doi:10.1038/143239a0. S2CID 4113262.
- ^ Schroeder, M. «Lise Meitner – Zur 125. Wiederkehr Ihres Geburtstages» (in German). Archived from the original on 19 July 2011. Retrieved 4 June 2009.
- ^ Crawford, E.; Sime, Ruth Lewin; Walker, Mark (1997). «A Nobel tale of postwar injustice». Physics Today. 50 (9): 26–32. Bibcode:1997PhT….50i..26C. doi:10.1063/1.881933.
- ^ Kullander, Sven (28 August 2001). «Accelerators and Nobel Laureates». Nobel Foundation. Archived from the original on 13 April 2008.
- ^ «The Nobel Prize in Physics 1990». Nobel Foundation. 17 October 1990. Archived from the original on 14 May 2008.
- ^ Demtröder, Wolfgang (2002). Atoms, Molecules and Photons: An Introduction to Atomic- Molecular- and Quantum Physics (1st ed.). Springer. pp. 39–42. ISBN 978-3-540-20631-6. OCLC 181435713.
- ^ Woan, Graham (2000). The Cambridge Handbook of Physics. Cambridge University Press. p. 8. ISBN 978-0-521-57507-2. OCLC 224032426.
- ^ Mohr, P.J.; Taylor, B.N. and Newell, D.B. (2014), «The 2014 CODATA Recommended Values of the Fundamental Physical Constants» Archived 11 February 2012 at the Wayback Machine (Web Version 7.0). The database was developed by J. Baker, M. Douma, and S. Kotochigova. (2014). National Institute of Standards and Technology, Gaithersburg, Maryland 20899.
- ^ MacGregor, Malcolm H. (1992). The Enigmatic Electron. Oxford University Press. pp. 33–37. ISBN 978-0-19-521833-6. OCLC 223372888.
- ^ a b Particle Data Group (2002). «The Particle Adventure». Lawrence Berkeley Laboratory. Archived from the original on 4 January 2007.
- ^ a b Schombert, James (18 April 2006). «Elementary Particles». University of Oregon. Archived from the original on 30 August 2011.
- ^ Jevremovic, Tatjana (2005). Nuclear Principles in Engineering. Springer. p. 63. ISBN 978-0-387-23284-3. OCLC 228384008.
- ^ Pfeffer, Jeremy I.; Nir, Shlomo (2000). Modern Physics: An Introductory Text. Imperial College Press. pp. 330–336. ISBN 978-1-86094-250-1. OCLC 45900880.
- ^ Wenner, Jennifer M. (10 October 2007). «How Does Radioactive Decay Work?». Carleton College. Archived from the original on 11 May 2008.
- ^ a b c Raymond, David (7 April 2006). «Nuclear Binding Energies». New Mexico Tech. Archived from the original on 1 December 2002.
- ^ Mihos, Chris (23 July 2002). «Overcoming the Coulomb Barrier». Case Western Reserve University. Archived from the original on 12 September 2006.
- ^ Staff (30 March 2007). «ABC’s of Nuclear Science». Lawrence Berkeley National Laboratory. Archived from the original on 5 December 2006.
- ^ Makhijani, Arjun; Saleska, Scott (2 March 2001). «Basics of Nuclear Physics and Fission». Institute for Energy and Environmental Research. Archived from the original on 16 January 2007.
- ^ Shultis, J. Kenneth; Faw, Richard E. (2002). Fundamentals of Nuclear Science and Engineering. CRC Press. pp. 10–17. ISBN 978-0-8247-0834-4. OCLC 123346507.
- ^ Fewell, M.P. (1995). «The atomic nuclide with the highest mean binding energy». American Journal of Physics. 63 (7): 653–658. Bibcode:1995AmJPh..63..653F. doi:10.1119/1.17828.
- ^ Mulliken, Robert S. (1967). «Spectroscopy, Molecular Orbitals, and Chemical Bonding». Science. 157 (3784): 13–24. Bibcode:1967Sci…157…13M. doi:10.1126/science.157.3784.13. PMID 5338306.
- ^ a b Brucat, Philip J. (2008). «The Quantum Atom». University of Florida. Archived from the original on 7 December 2006.
- ^ Manthey, David (2001). «Atomic Orbitals». Orbital Central. Archived from the original on 10 January 2008.
- ^ Herter, Terry (2006). «Lecture 8: The Hydrogen Atom». Cornell University. Archived from the original on 22 February 2012.
- ^ Bell, R.E.; Elliott, L.G. (1950). «Gamma-Rays from the Reaction H1(n,γ)D2 and the Binding Energy of the Deuteron». Physical Review. 79 (2): 282–285. Bibcode:1950PhRv…79..282B. doi:10.1103/PhysRev.79.282.
- ^ Smirnov, Boris M. (2003). Physics of Atoms and Ions. Springer. pp. 249–272. ISBN 978-0-387-95550-6.
- ^ Matis, Howard S. (9 August 2000). «The Isotopes of Hydrogen». Guide to the Nuclear Wall Chart. Lawrence Berkeley National Lab. Archived from the original on 18 December 2007.
- ^ Weiss, Rick (17 October 2006). «Scientists Announce Creation of Atomic Element, the Heaviest Yet». Washington Post. Archived from the original on 20 August 2011.
- ^ a b Sills, Alan D. (2003). Earth Science the Easy Way. Barron’s Educational Series. pp. 131–134. ISBN 978-0-7641-2146-3. OCLC 51543743.
- ^ Dumé, Belle (23 April 2003). «Bismuth breaks half-life record for alpha decay». Physics World. Archived from the original on 14 December 2007.
- ^ Lindsay, Don (30 July 2000). «Radioactives Missing From The Earth». Don Lindsay Archive. Archived from the original on 28 April 2007.
- ^ Tuli, Jagdish K. (April 2005). «Nuclear Wallet Cards». National Nuclear Data Center, Brookhaven National Laboratory. Archived from the original on 3 October 2011.
- ^ CRC Handbook (2002).
- ^ Krane, K. (1988). Introductory Nuclear Physics. John Wiley & Sons. pp. 68. ISBN 978-0-471-85914-7.
- ^ a b Mills, Ian; Cvitaš, Tomislav; Homann, Klaus; Kallay, Nikola; Kuchitsu, Kozo (1993). Quantities, Units and Symbols in Physical Chemistry (2nd ed.). Oxford: International Union of Pure and Applied Chemistry, Commission on Physiochemical Symbols Terminology and Units, Blackwell Scientific Publications. p. 70. ISBN 978-0-632-03583-0. OCLC 27011505.
- ^ Chieh, Chung (22 January 2001). «Nuclide Stability». University of Waterloo. Archived from the original on 30 August 2007.
- ^ «Atomic Weights and Isotopic Compositions for All Elements». National Institute of Standards and Technology. Archived from the original on 31 December 2006. Retrieved 4 January 2007.
- ^ Audi, G.; Wapstra, A.H.; Thibault, C. (2003). «The Ame2003 atomic mass evaluation (II)» (PDF). Nuclear Physics A. 729 (1): 337–676. Bibcode:2003NuPhA.729..337A. doi:10.1016/j.nuclphysa.2003.11.003. Archived (PDF) from the original on 16 October 2005.
- ^ Ghosh, D.C.; Biswas, R. (2002). «Theoretical calculation of Absolute Radii of Atoms and Ions. Part 1. The Atomic Radii». Int. J. Mol. Sci. 3 (11): 87–113. doi:10.3390/i3020087.
- ^ Shannon, R.D. (1976). «Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides» (PDF). Acta Crystallographica A. 32 (5): 751–767. Bibcode:1976AcCrA..32..751S. doi:10.1107/S0567739476001551. Archived (PDF) from the original on 14 August 2020. Retrieved 25 August 2019.
- ^ Dong, Judy (1998). «Diameter of an Atom». The Physics Factbook. Archived from the original on 4 November 2007.
- ^ Zumdahl, Steven S. (2002). Introductory Chemistry: A Foundation (5th ed.). Houghton Mifflin. ISBN 978-0-618-34342-3. OCLC 173081482. Archived from the original on 4 March 2008.
- ^ Bethe, Hans (1929). «Termaufspaltung in Kristallen». Annalen der Physik. 3 (2): 133–208. Bibcode:1929AnP…395..133B. doi:10.1002/andp.19293950202.
- ^ Birkholz, Mario (1995). «Crystal-field induced dipoles in heteropolar crystals – I. concept». Z. Phys. B. 96 (3): 325–332. Bibcode:1995ZPhyB..96..325B. CiteSeerX 10.1.1.424.5632. doi:10.1007/BF01313054. S2CID 122527743.
- ^ Birkholz, M.; Rudert, R. (2008). «Interatomic distances in pyrite-structure disulfides – a case for ellipsoidal modeling of sulfur ions» (PDF). Physica Status Solidi B. 245 (9): 1858–1864. Bibcode:2008PSSBR.245.1858B. doi:10.1002/pssb.200879532. S2CID 97824066. Archived (PDF) from the original on 2 May 2021. Retrieved 2 May 2021.
- ^ Birkholz, M. (2014). «Modeling the Shape of Ions in Pyrite-Type Crystals». Crystals. 4 (3): 390–403. doi:10.3390/cryst4030390.
- ^ Staff (2007). «Small Miracles: Harnessing nanotechnology». Oregon State University. Archived from the original on 21 May 2011. – describes the width of a human hair as 105 nm and 10 carbon atoms as spanning 1 nm.
- ^ Padilla, Michael J.; Miaoulis, Ioannis; Cyr, Martha (2002). Prentice Hall Science Explorer: Chemical Building Blocks. Upper Saddle River, New Jersey: Prentice-Hall, Inc. p. 32. ISBN 978-0-13-054091-1. OCLC 47925884.
There are 2,000,000,000,000,000,000,000 (that’s 2 sextillion) atoms of oxygen in one drop of water—and twice as many atoms of hydrogen.
- ^ «The Feynman Lectures on Physics Vol. I Ch. 1: Atoms in Motion». Archived from the original on 30 July 2022. Retrieved 3 May 2022.
- ^ a b «Radioactivity». Splung.com. Archived from the original on 4 December 2007. Retrieved 19 December 2007.
- ^ L’Annunziata, Michael F. (2003). Handbook of Radioactivity Analysis. Academic Press. pp. 3–56. ISBN 978-0-12-436603-9. OCLC 16212955.
- ^ Firestone, Richard B. (22 May 2000). «Radioactive Decay Modes». Berkeley Laboratory. Archived from the original on 29 September 2006.
- ^ Hornak, J.P. (2006). «Chapter 3: Spin Physics». The Basics of NMR. Rochester Institute of Technology. Archived from the original on 3 February 2007.
- ^ a b Schroeder, Paul A. (25 February 2000). «Magnetic Properties». University of Georgia. Archived from the original on 29 April 2007.
- ^ Goebel, Greg (1 September 2007). «[4.3] Magnetic Properties of the Atom». Elementary Quantum Physics. In The Public Domain website. Archived from the original on 29 June 2011.
- ^ Yarris, Lynn (Spring 1997). «Talking Pictures». Berkeley Lab Research Review. Archived from the original on 13 January 2008.
- ^ Liang, Z.-P.; Haacke, E.M. (1999). Webster, J.G. (ed.). Encyclopedia of Electrical and Electronics Engineering: Magnetic Resonance Imaging. Vol. 2. John Wiley & Sons. pp. 412–426. ISBN 978-0-471-13946-1.
- ^ Zeghbroeck, Bart J. Van (1998). «Energy levels». Shippensburg University. Archived from the original on 15 January 2005.
- ^ Fowles, Grant R. (1989). Introduction to Modern Optics. Courier Dover Publications. pp. 227–233. ISBN 978-0-486-65957-2. OCLC 18834711.
- ^ Martin, W.C.; Wiese, W.L. (May 2007). «Atomic Spectroscopy: A Compendium of Basic Ideas, Notation, Data, and Formulas». National Institute of Standards and Technology. Archived from the original on 8 February 2007.
- ^ «Atomic Emission Spectra – Origin of Spectral Lines». Avogadro Web Site. Archived from the original on 28 February 2006. Retrieved 10 August 2006.
- ^ Fitzpatrick, Richard (16 February 2007). «Fine structure». University of Texas at Austin. Archived from the original on 27 September 2011.
- ^ Weiss, Michael (2001). «The Zeeman Effect». University of California-Riverside. Archived from the original on 2 February 2008.
- ^ Beyer, H.F.; Shevelko, V.P. (2003). Introduction to the Physics of Highly Charged Ions. CRC Press. pp. 232–236. ISBN 978-0-7503-0481-8. OCLC 47150433.
- ^ Watkins, Thayer. «Coherence in Stimulated Emission». San José State University. Archived from the original on 12 January 2008. Retrieved 23 December 2007.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the «Gold Book») (1997). Online corrected version: (2006–) «valence». doi:10.1351/goldbook.V06588
- ^ Reusch, William (16 July 2007). «Virtual Textbook of Organic Chemistry». Michigan State University. Archived from the original on 29 October 2007.
- ^ «Covalent bonding – Single bonds». chemguide. 2000. Archived from the original on 1 November 2008.
- ^ Husted, Robert; et al. (11 December 2003). «Periodic Table of the Elements». Los Alamos National Laboratory. Archived from the original on 10 January 2008.
- ^ Baum, Rudy (2003). «It’s Elemental: The Periodic Table». Chemical & Engineering News. Archived from the original on 6 April 2011.
- ^ Goodstein, David L. (2002). States of Matter. Courier Dover Publications. pp. 436–438. ISBN 978-0-13-843557-8.
- ^ Brazhkin, Vadim V. (2006). «Metastable phases, phase transformations, and phase diagrams in physics and chemistry». Physics-Uspekhi. 49 (7): 719–724. Bibcode:2006PhyU…49..719B. doi:10.1070/PU2006v049n07ABEH006013. S2CID 93168446.
- ^ Myers, Richard (2003). The Basics of Chemistry. Greenwood Press. p. 85. ISBN 978-0-313-31664-7. OCLC 50164580.
- ^ Staff (9 October 2001). «Bose–Einstein Condensate: A New Form of Matter». National Institute of Standards and Technology. Archived from the original on 3 January 2008.
- ^ Colton, Imogen; Fyffe, Jeanette (3 February 1999). «Super Atoms from Bose–Einstein Condensation». The University of Melbourne. Archived from the original on 29 August 2007.
- ^ Jacox, Marilyn; Gadzuk, J. William (November 1997). «Scanning Tunneling Microscope». National Institute of Standards and Technology. Archived from the original on 7 January 2008.
- ^ «The Nobel Prize in Physics 1986». The Nobel Foundation. Archived from the original on 17 September 2008. Retrieved 11 January 2008. In particular, see the Nobel lecture by G. Binnig and H. Rohrer.
- ^ Jakubowski, N.; Moens, Luc; Vanhaecke, Frank (1998). «Sector field mass spectrometers in ICP-MS». Spectrochimica Acta Part B: Atomic Spectroscopy. 53 (13): 1739–1763. Bibcode:1998AcSpe..53.1739J. doi:10.1016/S0584-8547(98)00222-5.
- ^ Müller, Erwin W.; Panitz, John A.; McLane, S. Brooks (1968). «The Atom-Probe Field Ion Microscope». Review of Scientific Instruments. 39 (1): 83–86. Bibcode:1968RScI…39…83M. doi:10.1063/1.1683116.
- ^ Lochner, Jim; Gibb, Meredith; Newman, Phil (30 April 2007). «What Do Spectra Tell Us?». NASA/Goddard Space Flight Center. Archived from the original on 16 January 2008.
- ^ Winter, Mark (2007). «Helium». WebElements. Archived from the original on 30 December 2007.
- ^ Hinshaw, Gary (10 February 2006). «What is the Universe Made Of?». NASA/WMAP. Archived from the original on 31 December 2007.
- ^ Choppin, Gregory R.; Liljenzin, Jan-Olov; Rydberg, Jan (2001). Radiochemistry and Nuclear Chemistry. Elsevier. p. 441. ISBN 978-0-7506-7463-8. OCLC 162592180.
- ^ Davidsen, Arthur F. (1993). «Far-Ultraviolet Astronomy on the Astro-1 Space Shuttle Mission». Science. 259 (5093): 327–334. Bibcode:1993Sci…259..327D. doi:10.1126/science.259.5093.327. PMID 17832344. S2CID 28201406.
- ^ Lequeux, James (2005). The Interstellar Medium. Springer. p. 4. ISBN 978-3-540-21326-0. OCLC 133157789.
- ^ Smith, Nigel (6 January 2000). «The search for dark matter». Physics World. Archived from the original on 16 February 2008.
- ^ Croswell, Ken (1991). «Boron, bumps and the Big Bang: Was matter spread evenly when the Universe began? Perhaps not; the clues lie in the creation of the lighter elements such as boron and beryllium». New Scientist (1794): 42. Archived from the original on 7 February 2008.
- ^ Copi, Craig J.; Schramm, DN; Turner, MS (1995). «Big-Bang Nucleosynthesis and the Baryon Density of the Universe». Science (Submitted manuscript). 267 (5195): 192–199. arXiv:astro-ph/9407006. Bibcode:1995Sci…267..192C. doi:10.1126/science.7809624. PMID 7809624. S2CID 15613185. Archived from the original on 14 August 2019.
- ^ Hinshaw, Gary (15 December 2005). «Tests of the Big Bang: The Light Elements». NASA/WMAP. Archived from the original on 17 January 2008.
- ^ Abbott, Brian (30 May 2007). «Microwave (WMAP) All-Sky Survey». Hayden Planetarium. Archived from the original on 13 February 2013.
- ^ Hoyle, F. (1946). «The synthesis of the elements from hydrogen». Monthly Notices of the Royal Astronomical Society. 106 (5): 343–383. Bibcode:1946MNRAS.106..343H. doi:10.1093/mnras/106.5.343.
- ^ Knauth, D.C.; Knauth, D.C.; Lambert, David L.; Crane, P. (2000). «Newly synthesized lithium in the interstellar medium». Nature. 405 (6787): 656–658. Bibcode:2000Natur.405..656K. doi:10.1038/35015028. PMID 10864316. S2CID 4397202.
- ^ Mashnik, Stepan G. (2000). «On Solar System and Cosmic Rays Nucleosynthesis and Spallation Processes». arXiv:astro-ph/0008382.
- ^ Kansas Geological Survey (4 May 2005). «Age of the Earth». University of Kansas. Archived from the original on 5 July 2008.
- ^ a b Manuel (2001). Origin of Elements in the Solar System, pp. 407-430, 511-519
- ^ Dalrymple, G. Brent (2001). «The age of the Earth in the twentieth century: a problem (mostly) solved». Geological Society, London, Special Publications. 190 (1): 205–221. Bibcode:2001GSLSP.190..205D. doi:10.1144/GSL.SP.2001.190.01.14. S2CID 130092094. Archived from the original on 11 November 2007.
- ^ Anderson, Don L.; Foulger, G.R.; Meibom, Anders (2 September 2006). «Helium: Fundamental models». MantlePlumes.org. Archived from the original on 8 February 2007.
- ^ Pennicott, Katie (10 May 2001). «Carbon clock could show the wrong time». PhysicsWeb. Archived from the original on 15 December 2007.
- ^ Yarris, Lynn (27 July 2001). «New Superheavy Elements 118 and 116 Discovered at Berkeley Lab». Berkeley Lab. Archived from the original on 9 January 2008.
- ^ Diamond, H; et al. (1960). «Heavy Isotope Abundances in Mike Thermonuclear Device». Physical Review. 119 (6): 2000–2004. Bibcode:1960PhRv..119.2000D. doi:10.1103/PhysRev.119.2000.
- ^ Poston, John W. Sr. (23 March 1998). «Do transuranic elements such as plutonium ever occur naturally?». Scientific American. Archived from the original on 27 March 2015.
- ^ Keller, C. (1973). «Natural occurrence of lanthanides, actinides, and superheavy elements». Chemiker Zeitung. 97 (10): 522–530. OSTI 4353086.
- ^ Zaider, Marco; Rossi, Harald H. (2001). Radiation Science for Physicians and Public Health Workers. Springer. p. 17. ISBN 978-0-306-46403-4. OCLC 44110319.
- ^ «Oklo Fossil Reactors». Curtin University of Technology. Archived from the original on 18 December 2007. Retrieved 15 January 2008.
- ^ Weisenberger, Drew. «How many atoms are there in the world?». Jefferson Lab. Archived from the original on 22 October 2007. Retrieved 16 January 2008.
- ^ Pidwirny, Michael. «Fundamentals of Physical Geography». University of British Columbia Okanagan. Archived from the original on 21 January 2008. Retrieved 16 January 2008.
- ^ Anderson, Don L. (2002). «The inner inner core of Earth». Proceedings of the National Academy of Sciences. 99 (22): 13966–13968. Bibcode:2002PNAS…9913966A. doi:10.1073/pnas.232565899. PMC 137819. PMID 12391308.
- ^ Pauling, Linus (1960). The Nature of the Chemical Bond. Cornell University Press. pp. 5–10. ISBN 978-0-8014-0333-0. OCLC 17518275.
- ^ Anonymous (2 October 2001). «Second postcard from the island of stability». CERN Courier. Archived from the original on 3 February 2008.
- ^ Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. M.; et al. (2012). «Decay properties and stability of the heaviest elements» (PDF). International Journal of Modern Physics E. 21 (2): 1250013-1–1250013-20. Bibcode:2012IJMPE..2150013K. doi:10.1142/S0218301312500139. Archived (PDF) from the original on 3 December 2016. Retrieved 24 March 2020.
- ^ «Superheavy Element 114 Confirmed: A Stepping Stone to the Island of Stability». Berkeley Lab. 2009. Archived from the original on 20 July 2019. Retrieved 24 March 2020.
- ^ Möller, P. (2016). «The limits of the nuclear chart set by fission and alpha decay» (PDF). EPJ Web of Conferences. 131: 03002-1–03002-8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002. Archived (PDF) from the original on 11 March 2020. Retrieved 24 March 2020.
- ^ Koppes, Steve (1 March 1999). «Fermilab Physicists Find New Matter-Antimatter Asymmetry». University of Chicago. Archived from the original on 19 July 2008.
- ^ Cromie, William J. (16 August 2001). «A lifetime of trillionths of a second: Scientists explore antimatter». Harvard University Gazette. Archived from the original on 3 September 2006.
- ^ Hijmans, Tom W. (2002). «Particle physics: Cold antihydrogen». Nature. 419 (6906): 439–440. Bibcode:2002Natur.419..439H. doi:10.1038/419439a. PMID 12368837.
- ^ Staff (30 October 2002). «Researchers ‘look inside’ antimatter». BBC News. Archived from the original on 22 February 2007.
- ^ Barrett, Roger (1990). «The Strange World of the Exotic Atom». New Scientist (1728): 77–115. Archived from the original on 21 December 2007.
- ^ Indelicato, Paul (2004). «Exotic Atoms». Physica Scripta. T112 (1): 20–26. arXiv:physics/0409058. Bibcode:2004PhST..112…20I. doi:10.1238/Physica.Topical.112a00020. S2CID 11134265. Archived from the original on 4 November 2018.
- ^ Ripin, Barrett H. (July 1998). «Recent Experiments on Exotic Atoms». American Physical Society. Archived from the original on 23 July 2012.
Bibliography
- Oliver Manuel (2001). Origin of Elements in the Solar System: Implications of Post-1957 Observations. Springer. ISBN 978-0-306-46562-8. OCLC 228374906.
- Andrew G. van Melsen (2004) [1952]. From Atomos to Atom: The History of the Concept Atom. Translated by Henry J. Koren. Dover Publications. ISBN 0-486-49584-1.
- J.P. Millington (1906). John Dalton. J. M. Dent & Co. (London); E. P. Dutton & Co. (New York).
- Charles H. Holbrow; James N. Lloyd; Joseph C. Amato; Enrique Galvez; M. Elizabeth Parks (2010). Modern Introductory Physics. Springer Science & Business Media. ISBN 978-0-387-79079-4.
- John Dalton (1808). A New System of Chemical Philosophy vol. 1.
- John Dalton (1817). A New System of Chemical Philosophy vol. 2.
- John L. Heilbron (2003). Ernest Rutherford and the Explosion of Atoms. Oxford University Press. ISBN 0-19-512378-6.
- Jaume Navarro (2012). A History of the Electron: J. J. and G. P. Thomson. Cambridge University Press. ISBN 978-1-107-00522-8.
- Bernard Pullman (1998). The Atom in the History of Human Thought. Translated by Axel Reisinger. Oxford University Press. ISBN 0-19-511447-7.
Further reading
- Gangopadhyaya, Mrinalkanti (1981). Indian Atomism: History and Sources. Atlantic Highlands, New Jersey: Humanities Press. ISBN 978-0-391-02177-8. OCLC 10916778.
- Iannone, A. Pablo (2001). Dictionary of World Philosophy. Routledge. ISBN 978-0-415-17995-9. OCLC 44541769.
- King, Richard (1999). Indian philosophy: an introduction to Hindu and Buddhist thought. Edinburgh University Press. ISBN 978-0-7486-0954-3.
- McEvilley, Thomas (2002). The shape of ancient thought: comparative studies in Greek and Indian philosophies. Allworth Press. ISBN 978-1-58115-203-6.
- Siegfried, Robert (2002). From Elements to Atoms: A History of Chemical Composition. DIANE. ISBN 978-0-87169-924-4. OCLC 186607849.
- Teresi, Dick (2003). Lost Discoveries: The Ancient Roots of Modern Science. Simon & Schuster. pp. 213–214. ISBN 978-0-7432-4379-7. Archived from the original on 4 August 2020. Retrieved 25 October 2020.
- Wurtz, Charles Adolphe (1881). The Atomic Theory. New York: D. Appleton and company. ISBN 978-0-559-43636-9.
External links
- Sharp, Tim (8 August 2017). «What is an Atom?». Live Science.
- «Hitchhikers Guide to the Universe, Atoms and Atomic Structure». h2g2. BBC. 3 January 2006.
An atom is the basic unit of matter. All normal matter – everything that has mass – is made of atoms. This includes solids, liquids, and gases. The atom cannot be broken to parts by chemistry, so people once thought it was the smallest and simplest particle of matter.[1] There are over 100 different types of atoms, called chemical elements. Each type has the same basic structure, but a different number of parts.
Atoms are very small, but their exact size depends on the type. Atoms are from 0.1 to 0.5 nanometers across.[2] One nanometer is about 100,000 times smaller than the width of a human hair.[3] This makes one atom impossible to see without special tools. Scientists learn how they work by doing experiments.
Atoms are made of three types of subatomic particles. These are protons, neutrons, and electrons. Protons and neutrons have much more mass. They are in the middle of the atom, the nucleus. Lightweight electrons move quickly around them. The electromagnetic force holds the nucleus and electrons together.
Atoms with the same number of protons belong to the same chemical element. Examples of elements are carbon and gold. Atoms with the same number of protons, but different numbers of neutrons, are called isotopes. Usually an atom has the same number of electrons as protons. If an atom has more or less electrons than protons, it is called an ion, and has an electric charge.
Atoms can join by chemical bonds. Many things are made of more than one type of atom. These are chemical compounds or mixtures. A group of atoms connected by chemical bonds is called a molecule. For example, a water molecule is made of two hydrogen atoms and one oxygen atom. The forming or breaking of bonds is a chemical reaction.
Atoms split if the forces inside are too weak to hold them together. This is what causes radioactivity. Atoms can also join to make larger atoms at very high temperatures, such as inside a star. These changes are studied in nuclear physics. Most atoms on Earth are not radioactive. They are rarely made, destroyed, or changed into another type of atom.
History[change | change source]
The word «atom» comes from the Greek (ἀτόμος) «atomos», which means indivisible or uncuttable.[4] One of the first people to use the word «atom» is the Greek philosopher Democritus, around 400 BC. He thought that everything was made of particles called atoms. In his view, atoms moved in empty space, and they could not be divided into smaller pieces. Some Hindu, Jain, and Buddhist philosophers also had ideas like this.[5] Atomic theory was a mostly philosophical subject, with not much scientific investigation or study, until the early 1800s.[6]
In 1777 French chemist Antoine Lavoisier defined the term element as we use it today. He said that an element was any substance that could not be broken down into other substances by the methods of chemistry. Any substance which could be broken down was a compound.[7]
Dalton’s drawings of atoms (1808)
In 1803, English philosopher John Dalton suggested that elements were made of tiny, solid balls called atoms. Dalton believed that all atoms of the same element have the same mass. He said that compounds are formed when atoms of more than one element combine. According to Dalton, in a certain compound, the atoms of the compound’s elements always combine in the same way.[6][8]
In 1827, British scientist Robert Brown looked at pollen grains in water under his microscope. The pollen grains appeared to be shaking.[9] Brown used Dalton’s atomic theory to describe patterns in how they moved. This was called Brownian motion. In 1905 Albert Einstein used mathematics to prove that the pollen particles were being moved by the motion, or heat, of individual water molecules. By doing this, he proved that atoms are certainly real.[10][11]
In 1869, Russian scientist Dmitri Mendeleev published the first periodic table. The periodic table groups elements by their atomic number (how many protons they have; this is usually the same as the number of electrons). Elements in the same column, or period, usually have similar properties.[12] For example, helium, neon, argon, krypton, and xenon are all in the same column and have very similar properties. All these elements are gases that have no color or smell. Also, they cannot combine with other atoms to form compounds. Together they are known as noble gases.
The physicist J.J. Thomson was the first person to discover electrons. This happened while he was working with cathode rays in 1897. He realized they had a negative charge, and the rest of the atom had a positive charge. Thomson made the plum pudding model, which said that an atom was like plum pudding: the dried fruit (electrons) were stuck in a mass of pudding (having a positive charge).
In 1909, Ernest Rutherford used the Geiger–Marsden experiment to prove that most of an atom is in a very small space, the atomic nucleus. Rutherford took a photo plate and covered it with gold foil. He then shot alpha particles (made of two protons and two neutrons stuck together) at it. Many of the particles went through the gold foil, which proved that atoms are mostly empty space. Electrons are so small and fast-moving that they did not block the particles from going through. Rutherford later discovered protons in the nucleus.[13]
The Bohr model is not completely true, but it is useful for the idea of electron shells. This atom has 28 electrons in three shells.
In 1913, Niels Bohr introduced the Bohr model. This model showed that electrons travel around the nucleus in fixed circular orbits. This was better than the Rutherford model, but it was still not completely true. Improvements to the Bohr model have been made after it was first introduced.[14]
In 1925, chemist Frederick Soddy discovered that some elements had more than one kind of atom, called isotopes. Soddy believed that each different isotope of an element has a different mass.[15] To prove this, chemist Francis William Aston built the mass spectrometer, which measures the mass of individual atoms. Aston proved that Soddy was right. He also found that the mass of each atom is a whole number times the mass of the proton.[16] This meant that there must be some particles in the nucleus besides protons. In 1932, physicist James Chadwick shot alpha particles at beryllium atoms. He saw that a particle shot out of the beryllium atoms. This particle had no charge, but about the same mass as a proton. He named this particle the neutron.[17]
The best model so far comes from the Schrödinger equation. Schrödinger learned that the electrons exist in a cloud around the nucleus, called the electron cloud. In the electron cloud, it is impossible to know exactly where electrons are. The Schrödinger equation says where an electron is likely to be. This area is called the electron’s orbital.[18]
In 1937, German chemist Otto Hahn became the first person to make nuclear fission in a laboratory. He discovered this by chance when shooting neutrons at a uranium atom, hoping to make a new isotope. However, instead of a new isotope, the uranium changed into a barium atom, a smaller atom than uranium. Hahn had «broken» the uranium atom. This was the world’s first recorded nuclear fission reaction.[19] This discovery led to the creation of the atomic bomb and nuclear power, where fission happens over and over again, creating a chain reaction.
Later in the 20th century, physicists went deeper into the mysteries of the atom. Using particle accelerators, they discovered that protons and neutrons were made of other particles, called quarks.[20]
Structure and parts[change | change source]
Parts[change | change source]
This picture shows how small the nucleus is. The electrons are somewhere in the black cloud.
An atom is made of three main particles: the proton, the neutron, and the electron. Protons and neutrons have nearly the same size and mass (about 1.7×10−24 grams). The mass of an electron is about 1800 times smaller (about 9.1×10−28 grams). Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. Most atoms have no charge. The number of protons (positive) and electrons (negative) are the same, so the charges balance out to zero. However, ions have a different number of electrons than protons, so they have a positive or negative charge.[21][1]
Scientists believe that electrons are elementary particles: they are not made of any smaller pieces. Protons and neutrons are made of quarks of two types: up quarks and down quarks. A proton is made of two up quarks and one down quark, and a neutron is made of two down quarks and one up quark.[20]
Nucleus[change | change source]
The nucleus is in the middle of the atom. It is made of protons and neutrons. The nucleus makes up more than 99.9% of the mass of the atom. However, it is very small: about 1 femtometer (10−15 m) across, which is around 100,000 times smaller than the width of an atom, so it has a very high density. [22]
Usually in nature, two things with the same charge repel or shoot away from each other. So for a long time, scientists did not know how the positively charged protons in the nucleus stayed together. We now believe that the attraction between protons and neutrons comes from the strong nuclear force. This force also holds the quarks together that make up the protons and neutrons. Particles called mesons travel back and forth between protons and neutrons, and carry the force.[23][24]
A picture showing the main difficulty in nuclear fusion: Protons, which have positive charges, repel each other when forced together.
The number of neutrons in relation to protons defines whether the nucleus is stable or goes through radioactive decay. When there are too many neutrons or protons, the atom tries to make the numbers smaller or more equal by removing the extra particles. It sends out radiation in the form of alpha, beta, or gamma decay.[25] Nuclei can also change in other ways. Nuclear fission is when the nucleus breaks into two smaller nuclei, releasing a lot of energy. This release of energy makes nuclear fission useful for making bombs, and electricity in the form of nuclear power.[26]
The other way nuclei can change is through nuclear fusion, when two nuclei join or fuse to make a larger nucleus. This process requires very high amounts of energy to overcome the electric repulsion between the protons, as they have the same charge. Such high energies are most common in stars like our Sun, which fuses hydrogen for fuel. However, once fusion happens, far more energy is released because of the conversion of some of the mass into energy.[27]
The energy needed to break a nucleus into protons and neutrons is called its nuclear binding energy. This energy can be converted to mass according to Einstein’s famous formula E = mc2. Medium-sized nuclei, such as iron-56 and nickel-62, have the highest binding energy per proton or neutron, so they are the most stable. Very small and very large atoms have low binding energy. They can release energy through fission or fusion.[28]
Electrons[change | change source]
Electrons orbit, or travel around, the nucleus. They are called the atom’s electron cloud. They are attracted to the nucleus because of the electromagnetic force. Electrons have a negative charge, and the nucleus always has a positive charge, so they attract each other.[29]
According to the Bohr model, some electrons are farther from the nucleus than others in different layers. These are called electron shells.[29] Only the electrons in the outer shell can make chemical bonds. The number of electrons in the outer shell determines whether the atom is stable or which atoms it will bond with in a chemical reaction. If an atom has only one shell, it needs two electrons to be complete. Otherwise, the outer shell needs eight electrons to be complete.[30]
The Bohr model is important because it has the idea of energy levels. The electrons in each shell have a certain amount of energy. Shells that are farther from the nucleus have more energy. When a small burst of energy called a photon hits an electron, the electron can jump into a higher-energy shell. This photon must carry exactly the right amount of energy to bring the electron to the new energy level. A photon is a burst of light, and the amount of energy determines the color of light. So each type of atom will absorb certain colors of light, called the absorption spectrum. An electron can also send out, or emit, a photon, and fall into a lower energy shell. For the same reason, the atom will only send out certain colors of light, called the emission spectrum.[29]
The complete picture is more complicated. Unlike the Earth moving around the Sun, electrons do not move in a circle. We cannot know the exact place of an electron. We only know the probability, or chance, that it will be in any place. Each electron is part of an orbital, which describes where it is likely to be. No more than two electrons can be in one orbital; these two electrons have different spin.
Shapes of different orbitals around an atom
For each shell, numbered 1, 2, 3, and so on, there may be a number of different orbitals. These have different shapes, or point in different directions. Each orbital can be described by its three quantum numbers. The principal quantum number is the electron shell number. The azimuthal quantum number is represented by a letter: s, p, d, or f. Depending on the principal and azimuthal quantum numbers, the electron can have more or less energy. There is also a magnetic quantum number, but it does not usually affect the energy level. As more electrons are added, they join orbitals in order from lowest to highest energy. This order starts as follows: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d. For example, a chlorine atom has 17 electrons. So, it will have:
- 2 electrons in the 1s orbital
- 2 electrons in the 2s orbital
- 6 electrons in the 2p orbitals
- 2 electrons in the 3s orbital
- 5 electrons in the 3p orbitals
In other words, it has 2 electrons in the first shell, 8 in the second shell, and 7 in the third shell. [31]
Properties[change | change source]
Atomic number[change | change source]
The number of protons in an atom is called its atomic number. Atoms of the same element have the same atomic number. For example, all carbon atoms have six protons, so the atomic number of carbon is six.[32] Today, 118 elements are known. Depending on how the number is counted, 90 to 94 elements exist naturally on earth. All elements above number 94 have only been made by humans.[33] These elements are organized on the periodic table.
Atomic mass and weight[change | change source]
Because protons and neutrons have nearly the same mass, and the mass of electrons is very small, we can call the number of protons and neutrons in an atom its mass number. Most elements have several isotopes with different mass numbers. To name an isotope, we use the name of the element, followed by its mass number. So an atom with six protons and seven neutrons is called carbon-13.
Sometimes, we need a more exact measurement. The exact mass of an atom is called its atomic mass. This is usually measured with the atomic mass unit (amu), also called the dalton. One amu is exactly 1/12 of the mass of a carbon-12 atom, which is 1.7×10−24 grams. Hydrogen-1 has a mass of about 1 amu. The heaviest atom known, oganesson, has a mass of about 294 amu, or 4.9×10−22 grams.[34] The average mass of all atoms of a particular element is called its atomic weight.[32]
Size[change | change source]
The size of an atom depends on the size of its electron cloud. Moving down the periodic table, more electron shells are added. As a result, atoms get bigger. Moving to the right on the periodic table, more protons are added to the nucleus. This more positive nucleus pulls electrons more strongly, so atoms get smaller.[35] The biggest atom is caesium, which is about 0.596 nanometers wide according to one model. The smallest atom is helium, which is about 0.062 nanometers wide.[36]
How atoms interact[change | change source]
When atoms are far apart, they attract each other. This attraction is stronger for some kinds of atoms than others. At the same time, the heat, or kinetic energy, of atoms makes them always move. If the attraction is strong enough, relative to the amount of heat, atoms will form a solid. If the attraction is weaker, they will form a liquid, and if it is even weaker, they will form a gas.
Graphite is made of carbon atoms in layers. Covalent bonds hold each layer together. The attraction between different layers is a Van der Waals force.[37]
Chemical bonds are the strongest kinds of attraction between atoms. The movement of electrons explains all chemical bonds.
Atoms usually bond with each other in a way that fills or empties their outer electron shell. The most reactive elements have an almost full or almost empty outer shell. Atoms with a full outer shell, called noble gases, do not usually form bonds.[38]
There are three main types of bonds: ionic bonds, covalent bonds, and metallic bonds.
- In an ionic bond, one atom gives electrons to another atom. Each atom becomes an ion: an atom or group of atoms with a positive or negative charge. The positive ion (which has lost electrons) is called a cation; it is usually a metal. The negative ion (which has gained electrons) is called an anion; it is usually a nonmetal. Ionic bonding usually results in a regular network, or crystal, of ions held together.
- In a covalent bond, two atoms share electrons. This usually happens when both atoms are nonmetals. Covalent bonds often form molecules, ranging in size from two atoms to many more. They can also form large networks, such as glass or graphite. The number of bonds that an atom makes (its valency) is usually the number of electrons needed to fill its outer electron shell.
- In a metallic bond, electrons travel freely between many metal atoms. Any number of atoms can bond this way. Metals conduct electric current because electric charge can easily flow through them. Atoms in metals can move past each other, so it is easy to bend, stretch, and change the shape of metals.[39]
All atoms attract each other by Van der Waals forces. These forces are weaker than chemical bonds. They are caused when electrons move to one side of an atom. This movement gives a negative charge to that side. It also gives a positive charge to the other side. When two atoms line up their sides with negative and positive charges, they will attract.[40]
Although atoms are mostly empty space, they cannot pass through each other. When two atoms are very close, their electron clouds will repel each other by the electromagnetic force.[41]
Magnetism[change | change source]
To understand how magnets work, we can look at the properties of the atom. Any magnet has a north and south pole, and a certain strength. The direction and strength of a magnet, together, are called its magnetic moment. Every electron also has a magnetic moment, like a tiny magnet. This comes from the electron’s spin and its orbit around the nucleus. The magnetic moments for the electrons add up to a magnetic moment for the whole atom. This tells us how atoms act in a magnetic field.
Every electron has one of two opposite spins. We can think of one as turning to the right, and the other as turning to the left. If every electron is paired with an electron with the opposite spin in the same orbital, the magnetic moments will cancel out to zero. Atoms like this are called diamagnetic. They are only weakly repelled by a magnetic field.
However, if some electrons are not paired, the atom will have a lasting magnetic moment: it will be paramagnetic or ferromagnetic. When atoms are paramagnetic, the magnetic moment of each atom points in a random direction. They are weakly attracted to a magnetic field. When atoms are ferromagnetic, the magnetic moments of nearby atoms act on each other. They point in the same direction. This means that the whole object is a magnet, and it can point in the direction of a magnetic field. Ferromagnetic materials, such as iron, cobalt, and nickel, are strongly attracted to a magnetic field.[42]
Radioactive decay[change | change source]
An alpha particle shoots out of a nucleus.
Some elements, and many isotopes, have what is called an unstable nucleus. This means the nucleus is either too big to hold itself together, or it has too many protons or neutrons.[43] When a nucleus is unstable, it has to eliminate the excess mass of particles. It does this through radiation. An atom that does this is called radioactive. Unstable atoms emit radiation until they lose enough particles in the nucleus to become stable. All atoms above atomic number 82 (82 protons, lead) are radioactive.[44]
The nuclei in black are stable. All others are radioactive. The left axis is the number of protons, and the bottom axis is the number of neutrons.
There are three main types of radioactive decay: alpha, beta, and gamma.[25]
- Alpha decay is when the atom shoots out a particle having two protons and two neutrons. This is a helium-4 nucleus. The result is an element with an atomic number of two less than before. So, for example, if a uranium atom (atomic number 92) went through alpha decay, it would become thorium (atomic number 90). Alpha decay happens when an atom is too big and needs to get rid of some mass.
- Beta decay is when a neutron turns into a proton, or a proton turns into a neutron. In the first case, the atom shoots out an electron. In the second case, it is a positron (like an electron but with a positive charge). The result is an element with one higher or one lower atomic number than before. Beta decay happens when an atom has either too many protons or too many neutrons.
- Gamma decay is when an atom shoots out a gamma ray, or wave. It happens when there is a change in the energy of the nucleus. This is usually after a nucleus has gone through alpha or beta decay. There is no change in the atom’s mass, or atomic number, only in the stored energy inside the nucleus, in the form of particle spin.
Every radioactive element or isotope has a half-life. This is how long it takes half of any sample of atoms of that type to decay into a different isotope or element.[45]
Creation of atoms[change | change source]
Nearly all the hydrogen atoms in the Universe, most of the helium atoms, and some of the lithium atoms were made soon after the Big Bang. Even today, about 90% of all atoms in the Universe are hydrogen.[46]
All other atoms come from nuclear fusion in stars, or sometimes from cosmic rays that hit atoms. At the start of their life, all stars fuse hydrogen to make helium. The least massive stars, red dwarfs, are expected to stop there. All other stars will then fuse helium to make carbon and oxygen. In stars like the Sun, the temperature and pressure are too low to make larger atoms. But more massive stars continue fusion, until they create iron (atomic number 26) or nickel (atomic number 28). [47] Atoms can also grow larger when neutrons or protons hit them. This could happen inside stars or in supernovae. Most atoms on Earth were made by a star that existed before the Sun.[48]
People make very large atoms by smashing together smaller atoms in particle accelerators. However, these atoms often decay very quickly. Oganesson (element 118) has a half-life of 0.00089 seconds. Even larger atoms may be created in the future.[34]
[change | change source]
- Atomic physics, for more detail about the physics of atoms
- Atomic theory, for more detail about the history
- Chemistry, the field which studies all things that are made from atoms
- Exotic atom, an atom with different parts instead of protons, neutrons, and electrons
- Quantum mechanics, the study of small particles and how they interact with energy
- States of matter, the different forms in which atoms or molecules can be found
Sources[change | change source]
References[change | change source]
- ↑ 1.0 1.1 «What is an atom ?». NRC Web. March 19, 2020. Retrieved December 6, 2022.
- ↑ Philip, Michael; Dong, Judy (1998). Elert, Glenn (ed.). «Size of an Atom». The Physics Factbook. Archived from the original on January 30, 2022.
- ↑ Ley, Brian (1999). Elert, Glenn (ed.). «Diameter of a Human Hair». The Physics Factbook. Archived from the original on July 11, 2022.
- ↑ «Atom Definition & Meaning». Dictionary.com. Retrieved November 28, 2022.
- ↑ American Chemical Society 2010, pp. 21–33.
- ↑ 6.0 6.1 American Chemical Society 2010, pp. 1–5.
- ↑ Chalmers 2009, p. 168.
- ↑ Chalmers 2009, pp. 177–179.
- ↑ Chalmers 2009, p. 234.
- ↑ Lee, Y.K.; Hoon, Kelvin. «Brownian motion — a history». Archived from the original on December 18, 2007. Retrieved November 30, 2009.
- ↑ Chalmers 2009, p. 239.
- ↑ Flowers et al. 2019, pp. 165–169.
- ↑ Flowers et al. 2019, pp. 73–79.
- ↑ Flowers et al. 2019, pp. 131–135.
- ↑ «Frederick Soddy – Biographical». NobelPrize.org. Nobel Prize Outreach AB. Retrieved August 22, 2022.
- ↑ «Francis W. Aston – Biographical». NobelPrize.org. Nobel Prize Outreach AB. Retrieved August 7, 2022.
- ↑ American Chemical Society 2010, pp. 65–81.
- ↑ Orchin, Milton; Macomber, Roger S.; Pinhas, Allan; Wilson, R. Marshall (2005). «Atomic Orbital Theory» (PDF). The Vocabulary and Concepts of Organic Chemistry (2nd ed.). John Wiley & Sons, Inc.
- ↑ «Otto Hahn, Lise Meitner and Fritz Strassman». Science History Institute. December 7, 2017. Retrieved August 22, 2022.
- ↑ 20.0 20.1 Riordan, Michael (1992). «The Discovery of Quarks». Science. 256 (5061): 1287–1293. Bibcode:1992Sci…256.1287R. doi:10.1126/science.256.5061.1287. ISSN 0036-8075. JSTOR 2877300. PMID 17736758. S2CID 34363851 – via JSTOR.
- ↑ Flowers et al. 2019, p. 80.
- ↑ Flowers et al. 2019, p. 79.
- ↑ «Nobel Prize in Physics 1949 – Presentation Speech». NobelPrize.org. Nobel Prize Outreach AB. Retrieved May 13, 2022.
- ↑ Aoki, Sinya; Hatsuda, Tetsuo; Ishii, Noriyoshi (January 2010). «Theoretical Foundation of the Nuclear Force in QCD and Its Applications to Central and Tensor Forces in Quenched Lattice QCD Simulations». Progress of Theoretical Physics. 123 (1): 89–128. arXiv:0909.5585. Bibcode:2010PThPh.123…89A. doi:10.1143/PTP.123.89. S2CID 18840133.
- ↑ 25.0 25.1 Flowers et al. 2019, pp. 1086–1088.
- ↑ Flowers et al. 2019, pp. 1100–1105.
- ↑ Flowers et al. 2019, pp. 1110–1111.
- ↑ Iliadis 2007, pp. 33–34.
- ↑ 29.0 29.1 29.2 Flowers et al. 2019, pp. 128–135.
- ↑ Flowers et al. 2019, p. 215.
- ↑ Flowers et al. 2019, pp. 148–153.
- ↑ 32.0 32.1 Flowers et al. 2019, pp. 79–85.
- ↑ McMahon, Mary (July 27, 2022). «How Many Elements on the Periodic Table of the Elements Occur Naturally?». All the Science. Retrieved August 22, 2022.
- ↑ 34.0 34.1 «Oganesson | Og (Element) — PubChem». pubchem.ncbi.nlm.nih.gov. Retrieved August 6, 2022.
- ↑ Flowers et al. 2019, pp. 158–160.
- ↑
Clementi, E.; Raimond, D. L.; Reinhardt, W. P. (1967). «Atomic Screening Constants from SCF Functions. II. Atoms with 37 to 86 Electrons». Journal of Chemical Physics. 47 (4): 1300–1307. Bibcode:1967JChPh..47.1300C. doi:10.1063/1.1712084. - ↑ Chung, D. D. L. (2002). «Review Graphite». Journal of Materials Science. 37 (8): 1475–1489. doi:10.1023/A:1014915307738. S2CID 189839788.
- ↑ Reusch, William (July 16, 2007). «Virtual Textbook of Organic Chemistry». Michigan State University. Archived from the original on October 21, 2007.
- ↑ «Fundamentals of Chemical Bonding». LibreTexts. August 15, 2020. Retrieved May 18, 2022.
- ↑ Swinerd, Vicky (2003). «What are Van der Waals Forces?».
- ↑ Frank, Adam (April 7, 2015). «Why Doesn’t Your Butt Fall Through The Chair?». NPR.
- ↑ Serway, Moses & Moyer 1997, pp. 476–484.
- ↑ Serway, Moses & Moyer 1997, pp. 533–534.
- ↑ Sills, Alan D. (2003). Earth Science the Easy Way. Barron’s Educational Series. pp. 131–134. ISBN 978-0-7641-2146-3. OCLC 51543743.
- ↑ Flowers et al. 2019, p. 1090.
- ↑ Grochala, Wojciech (March 2015). «First there was hydrogen». Nature Chemistry. 7 (3): 264. Bibcode:2015NatCh…7..264G. doi:10.1038/nchem.2186. ISSN 1755-4349. PMID 25698337.
- ↑ Iliadis 2007, pp. 11–27.
- ↑ Iliadis 2007, pp. 568–570.
Bibliography[change | change source]
- Chalmers, Alan (2009). The scientist’s atom and the philosopher’s stone : how science succeeded and philosophy failed to gain knowledge of atoms. [Dordrecht]: Springer. ISBN 978-90-481-2362-9. OCLC 432702848.
- Flowers, Paul; Theopold, Klaus; Langley, Richard; Neth, Edward J.; Robinson, William R. (2019). Chemistry: Atoms First (2nd ed.). Houston, Texas: OpenStax, Rice University. ISBN 978-1-947172-63-0. OCLC 1089692119.
- Giunta, Carmen, ed. (2010). Atoms in chemistry : from Dalton’s predecessors to complex atoms and beyond. Washington, DC: American Chemical Society. ISBN 978-0-8412-2558-9. OCLC 659536310.
- Iliadis, Christian (2007). Nuclear Physics of Stars. Weinheim: Wiley-VCH. doi:10.1002/9783527618750. ISBN 978-3-527-40602-9.
- Serway, Raymond A.; Moses, Clement; Moyer, Curt A. (1997). Modern Physics (2nd ed.). Saunders College Publishing. ISBN 0-03-001547-2.
Other websites[change | change source]
Wikimedia Commons has media related to Atoms.
Wikiquote has a collection of quotations related to: Atom
- Atom (science) -Citizendium
- General information on atomic structure Archived December 23, 2012, at the Wayback Machine
- «A Brief History of the Atom». Archived from the original on December 9, 2009. Retrieved November 30, 2009.
- «Structure of Atom: Class 11 Chemistry NCERT Chapter 2». Reeii Education. May 30, 2020. Archived from the original on October 22, 2020. Retrieved October 18, 2020.
- «How does radioactive decay work?».
- «Atomic Emission Spectra — Origin of Spectral Lines». Archived from the original on February 28, 2006. Retrieved May 2, 2022.
- «S-Cool: Types of radiation».
English[edit]
Alternative forms[edit]
- atomus (obsolete)
Etymology[edit]
From Middle English attome, from Middle French athome, from Latin atomus (“smallest particle”), from Ancient Greek ἄτομος (átomos, “indivisible”), from ἀ- (a-, “not”) + τέμνω (témnō, “I cut”).
Pronunciation[edit]
- IPA(key): /ˈætəm/
- Rhymes: -ætəm
- Homophone: Adam (in dialects with flapping)
- Hyphenation: at‧om
Noun[edit]
atom (plural atoms)
- (chemistry, physics) The smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons. [from 16th c.]
- Meronyms: proton, neutron, electron
-
2013 September–October, Katie L. Burke, “In the news: Photosynthesis precursor”, in American Scientist[1], archived from the original on 13 April 2016:
-
Oxygen levels on Earth skyrocketed 2.4 billion years ago, when cyanobacteria evolved photosynthesis: the ability to convert water and carbon dioxide into carbohydrates and waste oxygen using solar energy. The evolutionary precursor of photosynthesis is still under debate, and a new study sheds light. The critical component of the photosynthetic system is the water-oxidizing complex, made up of manganese atoms and a calcium atom.
-
- (history of science) A hypothetical particle posited by Greek philosophers as an ultimate and indivisible component of matter. [from 15th c.]
- (now generally regarded figuratively) The smallest, indivisible constituent part or unit of something. [from 17th c.]
-
1835, John Ross; James Clark Ross, “Chapter XXXIV. Labour in Cutting through the Ice—Become Fixed for the Winter—Summary of the Month.”, in Narrative of a Second Voyage in Search of a North-west Passage, and of a Residence in the Arctic Regions, during the Years 1829, 1830, 1831, 1832, 1833; by Sir John Ross, C.B., K.S.A., K.C.S., &c. &c. Captain in the Royal Navy. Including the Reports of Commander (now Captain) J. C. Ross, R.N., F.R.S., F.L.S., &c. and the Discovery of the Northern Magnetic Pole, Philadelphia, Pa.: E. A. Carey & A. Hart; Baltimore, Md.: Carey, Hart & Co., →OCLC, pages 283–284:
-
Towards the following morning, the thermometer fell to 5°; and at daylight, there was not an atom of water to be seen in any direction.
-
-
1863, Charles Reade, Hard Cash[2]:
-
But at this critical moment the pirate astern sent a mischievous shot and knocked one of the men to atoms at the helm.
-
-
- (philosophy) In logical atomism, a fundamental fact that cannot be further broken down.
- (historical) The smallest medieval unit of time, equal to fifteen ninety-fourths of a second. [from 10th c.]
- A mote of dust in a sunbeam. [from 16th c.]
- A very small amount; a whit. [from 17th c.]
-
1873, “Pansy” [pseudonym; Isabella Macdonald Alden], “A Double Crisis”, in Three People, Cincinnati, Oh.: Western Tract and Book Society, 176 Elm Street, →OCLC, page 325:
-
«Doctor, tell me one word more,» said Theodore, quivering with suppressed emotion. «How do you think it will end?» / «I have hardly the faintest atom of hope,» answered this honest, earnest man.
-
-
1945 August 17, George Orwell [pseudonym; Eric Arthur Blair], chapter 1, in Animal Farm […], London: Secker & Warburg, →OCLC:
-
Now, comrades, what is the nature of this life of ours? Let us face it, our lives are miserable, laborious, and short. We are born, we are given just so much food as will keep the breath in our bodies, and those of us who are capable of it are forced to work to the last atom of our strength; and the very instant that our usefulness has come to an end we are slaughtered with hideous cruelty. No animal in England knows the meaning of happiness or leisure after he is a year old. No animal in England is free. The life of an animal is misery and slavery: that is the plain truth.
-
-
- (computing, programming, Lisp) An individual number or symbol, as opposed to a list; a scalar value. [from 20th c.]
- (mathematics) A non-zero member of a partially ordered set that has only zero below it (assuming that the poset has a least element, its «zero»). [from 20th c.]
- Antonym: coatom
- In a Venn diagram, an atom is depicted as an area circumscribed by lines but not cut by any line.
- (mathematics, set theory) An element of a set that is not itself a set; an urelement. [from 20th c.]
- (Canada, usually attributive) An age group division in hockey for nine- to eleven-year-olds.
Synonyms[edit]
- See also Thesaurus:atom
- (small amount): see also Thesaurus:modicum.
Derived terms[edit]
- atom bomb
- atom cocktail
- atom laser
- atom physics
- atom smasher
- atomic
- atomical
- atomically
- atomize
- atompunk
- Boolean atom
- exotic atom
- Gabor atom
- gram atom
- hadronic atom
- Hooke’s atom
- Rydberg atom
- social atom
- vortex atom
Descendants[edit]
- → Swahili: atomi
Translations[edit]
chemistry, physics: smallest possible amount of matter retaining its chemical properties
- Afrikaans: atoom (af)
- Albanian: atom (sq) m
- Amharic: አቶም (ʾätom)
- Arabic: ذَرَّة f (ḏarra)
- Armenian: ատոմ (hy) (atom)
- Assamese: পৰমাণু (pormanu), অণু (onu)
- Asturian: átomu (ast) m
- Azerbaijani: atom (az)
- Basque: atomo (eu)
- Belarusian: а́там m (átam)
- Bengali: পরমাণু (bn) (pormanu), অণু (bn) (onu)
- Breton: atom (br) m, atomenn (br) f
- Bulgarian: а́том (bg) m (átom)
- Burmese: အက်တမ် (my) (aktam), အဏုမြူ (my) (a.nu.mru)
- Catalan: àtom (ca) m
- Cherokee: ᎤᏍᏗ (usdi)
- Chinese:
- Cantonese: 原子 (jyun4 zi2)
- Mandarin: 原子 (zh) (yuánzǐ)
- Min Nan: 原子 (zh-min-nan) (goân-chú)
- Czech: atom (cs) m
- Danish: atom (da) n
- Dutch: atoom (nl) n
- Esperanto: atomo (eo)
- Estonian: aatom (et)
- Faroese: atom (fo) n
- Fiji Hindi: परमाणु ? (parmāṇu)
- Finnish: atomi (fi)
- French: atome (fr) m
- Galician: átomo (gl) m
- Georgian: ატომი (aṭomi)
- German: Atom (de) n
- Greek: άτομο (el) n (átomo)
- Guaraní: tumingue’a (gn)
- Gujarati: પરમાણુ m or n (parmāṇu)
- Hebrew: אָטוֹם (he) m (atom)
- Hindi: परमाणु (hi) m (parmāṇu), अणु (hi) m (aṇu)
- Hungarian: atom (hu)
- Icelandic: frumeind (is) f, atóm (is) n
- Ido: atomo (io)
- Indonesian: atom (id)
- Irish: adamh (ga) m
- Italian: atomo (it) m
- Japanese: 原子 (ja) (げんし, genshi), アトム (atomu)
- Javanese: ꦥꦫꦩꦤꦸ (paramanu)
- Kannada: ಪರಮಾಣು (kn) (paramāṇu)
- Kazakh: атом (kk) (atom)
- Khmer: បរមាណូ (km) (paʼraʼmaanou), អាតូម (ʼaatoum)
- Korean: 원자(原子) (ko) (wonja), 아톰 (atom)
- Kurdish:
- Central Kurdish: ئەتۆم (ckb) (etom)
- Kyrgyz: атом (ky) (atom)
- Lao: ປະລະມະນູ (pa la ma nū), ອາຕົມ (lo) (ʼā tom)
- Latin: atomus f
- Latvian: atoms m
- Lithuanian: atomas (lt) m
- Luxembourgish: Atom (lb) m
- Macedonian: атом m (atom)
- Malay: atom (ms)
- Malayalam: അണു (ml) (aṇu)
- Manx: breneen f
- Maori: ngota
- Marathi: अणू (mr) m (aṇū)
- Mongolian: атом (mn) (atom)
- Nahuatl: nantzintetl
- Nepali: अणु (ne) (aṇu)
- Newar: अणु (aṇu)
- Norman: atonme m
- Norwegian:
- Bokmål: atom (no) n
- Nynorsk: atom n
- Occitan: atòm (oc) m, atòme m
- Pashto: اټوم m (aṭóm)
- Persian: اتم (fa) (atom)
- Plautdietsch: Atomm m
- Polish: atom (pl) m
- Portuguese: átomo (pt) m
- Quechua: iñuku
- Romanian: atom (ro) m
- Russian: а́том (ru) m (átom)
- Sanskrit: परमाणु (sa) m (paramāṇu), अणु (sa) m (aṇu)
- Scottish Gaelic: dadam m
- Serbo-Croatian:
- Cyrillic: а̀то̄м m
- Roman: àtōm (sh) m
- Sinhalese: පරමාණු (paramāṇu)
- Slovak: atóm (sk) m
- Slovene: atom (sl) m
- Southern Ndebele: imbumbulo ?
- Spanish: átomo (es) m
- Swahili: atomi class 9/10
- Swedish: atom (sv) c
- Tagalog: atomo (tl)
- Tajik: атом (tg) (atom)
- Tamil: அணு (ta) (aṇu)
- Telugu: అణువు (te) (aṇuvu)
- Thai: ปรมาณู (th) (bpà-rá-maa-nuu), อะตอม (th) (à-dtɔm)
- Tibetan: རྡུལ་ཕྲན (rdul phran)
- Turkish: atom (tr)
- Turkmen: atom (tk)
- Ukrainian: а́том (uk) m (átom), не́ділка (nédilka)
- Urdu: ایٹم (ur) m (eṭam)
- Uyghur: ئاتوم (atom)
- Uzbek: atom (uz)
- Vietnamese: nguyên tử (vi) (原子)
- Volapük: taum (vo)
- Welsh: atom (cy) m or f
- Wolof: xarefulwoon
- Yiddish: אַטאָם m (atom)
- Zulu: umsukantozonke ?
historical: theoretical particle of matter
- Afrikaans: atoom (af)
- Arabic: ذَرَّة f (ḏarra)
- Armenian: ատոմ (hy) (atom)
- Catalan: àtom (ca) m
- Chinese:
- Mandarin: 原子 (zh) (yuánzǐ)
- Czech: atom (cs) m
- Danish: atom (da) n
- Dutch: atoom (nl) n
- Finnish: atomi (fi)
- French: atome (fr) m
- Galician: átomo (gl) m
- German: Atom (de) n
- Greek: άτομο (el) n (átomo)
- Ancient: ἄτομον n (átomon)
- Hawaiian: hunaʻiʻo
- Hebrew: אָטוֹם (he) m (atom)
- Hindi: परमाणु (hi) m (parmāṇu)
- Irish: adamh (ga) m
- Italian: atomo (it) m
- Japanese: 元素 (ja) (げんそ, genso)
- Khmer: បរមាណូ (km) (paʼraʼmaanou)
- Korean: 원자(原子) (ko) (wonja)
- Kurdish:
- Central Kurdish: ئەتۆم (ckb) (etom)
- Latvian: atoms m
- Manx: breneen f
- Maori: ngota
- Marathi: अणू (mr) ? (aṇū)
- Norwegian:
- Bokmål: atom (no) n
- Portuguese: átomo (pt) m
- Russian: а́том (ru) m (átom)
- Sanskrit: परमाणु (sa) m (paramāṇu), अणु (sa) m (aṇu)
- Spanish: átomo (es) m
- Swedish: atom (sv) c
- Tagalog: atomo (tl)
- Thai: ปรมาณู (th) (bpà-rá-maa-nuu), อะตอม (th) (à-dtɔm)
smallest indivisible constituent
- Bulgarian: атом (bg) m (atom)
- Danish: atom (da) n
- Finnish: perusosanen
- Galician: átomo (gl) m
- Irish: adamh (ga) m
- Italian: atomo (it) m
- Japanese: 単位 (ja) (たんい, tan’i)
- Marathi: लघुत्तम अविभाज्य घटक ? (laghuttam avibhājya ghaṭak)
- Norwegian:
- Bokmål: atom (no) n
- Portuguese: átomo (pt) m
- Tagalog: atomo (tl)
Translations to be checked
- Bengali: (please verify) অণু (bn) (onu)
- Breton: (please verify) atom (br) ? (collective noun), (please verify) atomenn (br) f sg
- Interlingua: (please verify) atomo (ia)
- Romanian: (please verify) atom (ro) m
- Telugu: (please verify) అణువు (te) (aṇuvu)
- Thai: (please verify) อะตอม (th) (à-dtɔm)
See also[edit]
- ⚛
- chemical element
Further reading[edit]
atom on Wikipedia.Wikipedia
Anagrams[edit]
- Amto, Mato, Mota, TMAO, atmo, moat, mota, toma
Albanian[edit]
Noun[edit]
atom m (definite singular atomi)
- (physics, chemistry) atom
Further reading[edit]
- “atom” on fjalorthi.com
- atom in Fjalor i gjuhës së sotme shqipe at shkenca.org
Breton[edit]
Pronunciation[edit]
- IPA(key): /ˈa.tɔmː/
Noun[edit]
atom m (collective, plural atomennoù, singulative atomenn)
- (physics) atoms
Derived terms[edit]
- atomek
Czech[edit]
Pronunciation[edit]
- IPA(key): [ˈatom]
Noun[edit]
atom m
- (physics) atom
[edit]
- atomický
- atomový
Further reading[edit]
- atom in Příruční slovník jazyka českého, 1935–1957
- atom in Slovník spisovného jazyka českého, 1960–1971, 1989
Danish[edit]
Etymology[edit]
Via German Atom n and Latin atomus f from Ancient Greek ἄτομοι (φύσεις) f (átomoi (phúseis)), ἄτομα (σώματα) n (átoma (sṓmata), “indivisible particles of matter”).
Pronunciation[edit]
- IPA(key): [aˈtˢoˀm]
Noun[edit]
atom n (singular definite atomet, plural indefinite atomer)
- atom
Declension[edit]
Hungarian[edit]
Etymology[edit]
From English atom, from Ancient Greek ἄτομος (átomos, “indivisible”), from ἀ- (a-, “not”) + τέμνω (témnō, “I cut”).[1]
Pronunciation[edit]
- IPA(key): [ˈɒtom]
- Hyphenation: atom
- Rhymes: -om
Noun[edit]
atom (plural atomok)
- atom
Declension[edit]
| Inflection (stem in -o-, back harmony) | ||
|---|---|---|
| singular | plural | |
| nominative | atom | atomok |
| accusative | atomot | atomokat |
| dative | atomnak | atomoknak |
| instrumental | atommal | atomokkal |
| causal-final | atomért | atomokért |
| translative | atommá | atomokká |
| terminative | atomig | atomokig |
| essive-formal | atomként | atomokként |
| essive-modal | — | — |
| inessive | atomban | atomokban |
| superessive | atomon | atomokon |
| adessive | atomnál | atomoknál |
| illative | atomba | atomokba |
| sublative | atomra | atomokra |
| allative | atomhoz | atomokhoz |
| elative | atomból | atomokból |
| delative | atomról | atomokról |
| ablative | atomtól | atomoktól |
| non-attributive possessive — singular |
atomé | atomoké |
| non-attributive possessive — plural |
atoméi | atomokéi |
| Possessive forms of atom | ||
|---|---|---|
| possessor | single possession | multiple possessions |
| 1st person sing. | atomom | atomjaim |
| 2nd person sing. | atomod | atomjaid |
| 3rd person sing. | atomja | atomjai |
| 1st person plural | atomunk | atomjaink |
| 2nd person plural | atomotok | atomjaitok |
| 3rd person plural | atomjuk | atomjaik |
Derived terms[edit]
- atomi
- atomnyi
- atomos
- atomarzenál
- atomágyú
- atombomba
- atomburok
- atomcsend
- atomcsoport
- atomegyezmény
- atomelmélet
- atomenergia
- atomerőmű
- atomfegyver
- atomfizika
- atomfizikus
- atomhajtású
- atomhatalom
- atomháború
- atommag
- atommodell
- atomóra
- atompálya
- atomprogram
- atomreaktor
- atomrobbanás
- atomrobbantás
- atomsugár
- atomsúly
- atomszám
- atomszemét
- atomszerkezet
- atomtemető
- atomtömeg
- atomtudós
- bóratom
- gázatom
- héliumatom
- hidrogénatom
- kénatom
- klóratom
- nitrogénatom
- oxigénatom
- szénatom
References[edit]
- ^ Tótfalusi, István. Idegenszó-tár: Idegen szavak értelmező és etimológiai szótára (’A Storehouse of Foreign Words: an explanatory and etymological dictionary of foreign words’). Budapest: Tinta Könyvkiadó, 2005. →ISBN
Further reading[edit]
- atom in Bárczi, Géza and László Országh. A magyar nyelv értelmező szótára (‘The Explanatory Dictionary of the Hungarian Language’, abbr.: ÉrtSz.). Budapest: Akadémiai Kiadó, 1959–1962. Fifth ed., 1992: →ISBN
- atom in Ittzés, Nóra (ed.). A magyar nyelv nagyszótára (’A Comprehensive Dictionary of the Hungarian Language’). Budapest: Akadémiai Kiadó, 2006–2031 (work in progress; published A–ez as of 2023)
Indonesian[edit]
Etymology[edit]
Internationalism, borrowed from Dutch atoom (“atom”), from French atome, from Latin atomus, from Ancient Greek ἄτομος (átomos).
Pronunciation[edit]
- IPA(key): /ˈatɔm/
- Rhymes: -tɔm, -ɔm, -m
- Hyphenation: a‧tom
Noun[edit]
atom (plural atom—atom, first-person possessive atomku, second-person possessive atommu, third-person possessive atomnya)
- (chemistry, nuclear physics) atom, the smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons.
- (figurative) modern
- Synonyms: modern, mutakhir
- (figurative) plastic, a synthetic, solid, hydrocarbon-based polymer, whether thermoplastic or thermosetting.
- Synonym: plastik
Derived terms[edit]
- pengatoman
- seatom
Further reading[edit]
- “atom” in Kamus Besar Bahasa Indonesia, Jakarta: Language Development and Fostering Agency — Ministry of Education, Culture, Research, and Technology of the Republic Indonesia, 2016.
Malay[edit]
Etymology[edit]
From English atom, from Old French atome, from Latin atomus, from Ancient Greek ἄτομος (átomos).
Pronunciation[edit]
- IPA(key): /atom/
- Rhymes: -atom, -tom, -om
Noun[edit]
atom (plural atom—atom, informal 1st possessive atomku, 2nd possessive atommu, 3rd possessive atomnya)
- (physics) atom (physics: smallest possible amount of matter retaining its chemical properties)
Norwegian Bokmål[edit]
Etymology[edit]
From Ancient Greek ἄτομος (átomos, “indivisible, uncut, undivided”), both from ἀ- (a-, “not, without”), from Proto-Hellenic *ə- (“un-, not; without, lacking”), from Proto-Indo-European *n̥- (“not, un-”) + and from τέμνω (témnō, “I cut, hew, wound, butcher”), from Proto-Indo-European *tm̥-n-h₁-, from *temh₁- (“to cut”).
Pronunciation[edit]
- IPA(key): /aˈtuːm/
- Rhymes: -uːm
- Hyphenation: at‧om
- Homophone: atom-
Noun[edit]
atom n (definite singular atomet, indefinite plural atom or atomer, definite plural atoma or atomene)
- (chemistry, physics) an atom (the smallest possible amount of matter which still retains its identity as a chemical element, now known to consist of a nucleus surrounded by electrons)
-
1943, Carl Fred. Holmboe, Michael Faraday, page 94:
-
tinnklorid … består av et atom tinn og to atomer klor
- tin chloride… consists of one atom of tin and two atoms chlorine tin chloride… consists of one atom of tin and two atoms of chlorine
-
-
1943, Carl Fred. Holmboe, Michael Faraday, page 164:
-
man var nådd frem til dets minste byggesten. Denne kalte Demokritos et atom: ἄτομος som betyr udelelig
- one had reached its smallest building block. This one called Democritus an atom: ἄτομος which means indivisible
-
-
1951, Agnar Mykle, Morgen i appelsingult, page 42:
-
det var nok atomene [som har forårsaket katastrofen] likevel, som jeg trodde!
- it was probably the atoms [that caused the disaster] anyway, as I thought!
-
-
2014, Nasjonal digital læringsarena[ndla.no]:
-
for å forstå hvordan stoffer reagerer med hverandre, og hvorfor de ulike stoffene har forskjellige egenskaper, må vi først lære om de minste byggesteinene i naturen, nemlig atomer
- to understand how substances react with each other, and why the different substances have different properties, we must first learn about the smallest building blocks in nature, namely atoms
-
-
et atom består av en atomkjerne omgitt av elektroner
- an atom consists of an atomic nucleus surrounded by electrons
-
- (figuratively) an atom (the smallest, indivisible constituent part or unit of something)
-
1865, H. Schulze, Fra Lofoten og Solør, page 87:
-
[stokken] maatte styrte udover ham og knuse ham til atomer
- [the stick] had to crash over him and crush him into atoms
-
-
1891, Arne Garborg, Trætte Mænd, page 230:
-
i samvittighedsnaget en draabe smigret forfængelighed – Og i forfængeligheden et atom selvforagt
- in the gnaw of conscience a drop of flattered vanity — And in vanity an atom of self-loathing
-
-
1910, Sven Elvestad, Angsten, page 29:
-
i et lidet atom af tid synes han fremdeles han er et andet og fjernt sted
- in a small atom of time he still thinks he is another and distant place
-
- Synonyms: grann, partikkel, smule
-
Derived terms[edit]
- antiatom
- atom-
- atomaktivist
- atomalder
- atomammunisjon
- atomangrep
- atomanlegg
- atomar
- atomartilleri
- atomaske
- atomavfall
- atomavrustning
- atomavtale
- atombase
- atombedrift
- atombombe
- atombombefly
- atombombeforsøk
- atombombemål
- atombombeprøve
- atombombestøv
- atombrennstoff
- atombrensel
- atombryter
- atombyrå
- atombåt
- atomdiplomat
- atomdiplomati
- atomdrevet
- atomdrift
- atomdrivstoff
- atomekspert
- atomeksplosjon
- atomenergi
- atomfly
- atomforsker
- atomforskning
- atomforsvar
- atomfred
- atomfri
- atomfrykt
- atomfysiker
- atomfysikk
- atomgitter
- atomgranat
- atomhemmelighet
- atomhode
- atomild
- atomindustri
- atomingeniør
- atominstitutt
- atomisere
- atomisk
- atomisme
- atomistisk
- atomisør
- atomkampanje
- atomkanon
- atomkappløp
- atomkirkegård
- atomkjerne
- atomklokke
- atomklubb
- atomkonferanse
- atomkontroll
- atomkraft
- atomkraftverk
- atomkrig
- atomladet
- atomladning
- atommakt
- atommarsj
- atommasse
- atommasseenhet
- atommile
- atommodell
- atommotor
- atommylder
- atommyndighet
- atommål
- atomnasjon
- atomnedfall
- atomnedruste
- atomnedrustning
- atomnummer
- atomopprustning
- atomorbital
- atomparaply
- atompolitikk
- atomproduksjon
- atomprosjekt
- atomprosjektil
- atomprotest
- atomprotestant
- atomprøve
- atomsibryter
- atomspaltning
- atomvekt
- atomvæpnet
- atomær
- donoratom
- fremmedatom
- gramatom
- heteroatom
- hydrogenatom
- oksygenatom
- sentralatom
- strålingsatom
- uranatom
[edit]
- atom- (prefix)
References[edit]
- “atom” in The Bokmål Dictionary.
- “atom” in Det Norske Akademis ordbok (NAOB).
- “atom” in Store norske leksikon
- “atom (historikk)” in Store norske leksikon
- “atom (atomteori)” in Store norske leksikon
Anagrams[edit]
- atom-, mota
Norwegian Nynorsk[edit]
Etymology[edit]
From Ancient Greek ἄτομος (átomos).
Noun[edit]
atom n (definite singular atomet, indefinite plural atom, definite plural atoma)
- an atom
Derived terms[edit]
- atommasse
- atomvekt
[edit]
- atom- (prefix)
References[edit]
- “atom” in The Nynorsk Dictionary.
Old Irish[edit]
Etymology[edit]
Borrowed from Latin atomus.
Pronunciation[edit]
- IPA(key): /ˈadoṽ/
Noun[edit]
atom m
- atom, mote
Inflection[edit]
| Masculine o-stem | |||
|---|---|---|---|
| Singular | Dual | Plural | |
| Nominative | atom | atomL | atoimL |
| Vocative | atoim | atomL | atomuH |
| Accusative | atomN | atomL | atomuH |
| Genitive | atoimL | atom | atomN |
| Dative | atomL | atomaib | atomaib |
Initial mutations of a following adjective:
|
Descendants[edit]
- Middle Irish: atam
- Irish: adamh
Polish[edit]
Etymology[edit]
Borrowed from French atome, from Latin atomus, from Ancient Greek ἄτομος (átomos).
Pronunciation[edit]
- IPA(key): /ˈa.tɔm/
- Rhymes: -atɔm
- Syllabification: a‧tom
Noun[edit]
atom m inan
- (physics) atom [+genitive = of what]
- nuclear device (something that operates thanks to nuclear energy)
Declension[edit]
Derived terms[edit]
- antyatomowy
- atomistyczny
- atomowy
- bezatomowy
- przeciwatomowy
- atomistycznie
- atomowo
- przeciwatomowo
- antyatom
- atomista
- atomistyka
- atomizacja
- atomizator
- atomizer
- atomizm
- atomowiec
- atomówka
- atomizować
- zatomizować
Further reading[edit]
- atom in Wielki słownik języka polskiego, Instytut Języka Polskiego PAN
- atom in Polish dictionaries at PWN
Romanian[edit]
Etymology[edit]
From French atome, from Latin atomus.
Pronunciation[edit]
- IPA(key): /aˈtom/
Noun[edit]
atom m (plural atomi)
- atom
Declension[edit]
Declension of atom
| singular | plural | |||
|---|---|---|---|---|
| indefinite articulation | definite articulation | indefinite articulation | definite articulation | |
| nominative/accusative | (un) atom | atomul | (niște) atomi | atomii |
| genitive/dative | (unui) atom | atomului | (unor) atomi | atomilor |
| vocative | atomule | atomilor |
Further reading[edit]
- atom in DEX online — Dicționare ale limbii române (Dictionaries of the Romanian language)
Serbo-Croatian[edit]
Etymology[edit]
From Ancient Greek ἄτομος (átomos).
Pronunciation[edit]
- IPA(key): /ǎtoːm/
- Hyphenation: a‧tom
Noun[edit]
àtōm m (Cyrillic spelling а̀то̄м)
- atom
Declension[edit]
References[edit]
- “atom” in Hrvatski jezični portal
Swedish[edit]
Noun[edit]
atom c
- atom; the smallest particle to retain the properties of the element
- (historical) atom; the theoretically smallest possible particle
Declension[edit]
| Declension of atom | ||||
|---|---|---|---|---|
| Singular | Plural | |||
| Indefinite | Definite | Indefinite | Definite | |
| Nominative | atom | atomen | atomer | atomerna |
| Genitive | atoms | atomens | atomers | atomernas |
[edit]
- atombomb
- atomisk
- atomkraft
- atomkärna
- atomvapen
- atomär
See also[edit]
- elektron
- elementarpartikel
- foton
- neutron
- positron
Turkish[edit]
Etymology[edit]
Borrowed from French atome.
Pronunciation[edit]
- IPA(key): [ɑˈtɔm]
Noun[edit]
atom (definite accusative atomu, plural atomlar)
- (physics) atom
- a kind of sugary drink common in and around Mersin province
Declension[edit]
| Inflection | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominative | atom | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Definite accusative | atomu | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Singular | Plural | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Nominative | atom | atomlar | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Definite accusative | atomu | atomları | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dative | atoma | atomlara | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Locative | atomda | atomlarda | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ablative | atomdan | atomlardan | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Genitive | atomun | atomların | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Derived terms[edit]
- atom bombası
[edit]
- atomik
References[edit]
- Nişanyan, Sevan (2002–), “atom”, in Nişanyan Sözlük
1
a
: the smallest particle of an element that can exist either alone or in combination
b
: the atom considered as a source of vast potential constructive or destructive energy
… a largely forgotten legacy of this country’s conquest of the atom.—
… when Congress passed the Atomic Energy Act in 1954 and allowed private utilities to «harness the atom.»—
2
: a tiny particle : bit
There’s not an atom of truth in what he said.
3
: one of the minute indivisible particles of which according to ancient materialism (see materialism sense 1a) the universe is composed
Did you know?
Some ancient philosophers believed that matter is infinitely divisible, that any particle, no matter how small, can always be divided into smaller particles. Others believed that there must be a limit and that everything in the universe must be made up of tiny indivisible particles. Such a hypothetical particle was called atomos in Greek, which means “indivisible.” According to modern atomic theory, all matter is made up of tiny particles named atoms from the ancient Greek atomos. However, it has turned out that atoms are not indivisible after all. Indeed, the splitting of atoms can be used to produce vast amounts of energy, as in atom bombs.
Synonyms
Example Sentences
There is not an atom of truth to what he said.
give me just one atom of information about the novel’s surprise ending
Recent Examples on the Web
An atom, for example, happens when there is enough energy in the quark fields to create quarks that don’t get canceled out by anti-matter quarks (though no one is sure why).
—
Hydrogen from this influx could have combined with oxygen atoms in the beads to form water.
—
When these excited atoms eventually return to their normal ground state, the excess energy from the collision shoots off in the form of vibrant light.
—
Did a tiny amino acid, a cluster of carbon, hydrogen, nitrogen, oxygen and sulfur atoms, kill Beethoven, and not syphilis or lead poisoning, as previously proposed?
—
These atoms can be cooled close to absolute zero in a lattice grid of laser light, reducing noise and improving timing stability.
—
In a semiconductor with a wide bandgap, the bonds between atoms are strong and so the material is usually able to withstand relatively high voltages before the bonds break and the transistor is said to break down.
—
Some of the layers of materials used to build semiconductors are only an atom thick, meaning they cannot be shrunk further.
—
Moreover, the short, femtosecond x-ray pulses work like a high-speed camera, helping researchers capture ultrafast processes such as the movement of electrons and atoms.
—
See More
These examples are programmatically compiled from various online sources to illustrate current usage of the word ‘atom.’ Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors. Send us feedback about these examples.
Word History
Etymology
Middle English, from Latin atomus, from Greek atomos, from atomos indivisible, from a- + temnein to cut
First Known Use
15th century, in the meaning defined at sense 3
Time Traveler
The first known use of atom was
in the 15th century
Dictionary Entries Near atom
Cite this Entry
“Atom.” Merriam-Webster.com Dictionary, Merriam-Webster, https://www.merriam-webster.com/dictionary/atom. Accessed 13 Apr. 2023.
Share
More from Merriam-Webster on atom
Last Updated:
12 Apr 2023
— Updated example sentences
Subscribe to America’s largest dictionary and get thousands more definitions and advanced search—ad free!
Merriam-Webster unabridged
Last Updated: April 20, 2022 | Author: howto-Trust
Contents
- 1 What does the word atom actually mean?
- 2 What does the Greek meaning of atom mean?
- 3 What is the meaning of atom example?
- 4 What is an atom in simple words?
- 5 Are humans made of atoms?
- 6 How do you explain an atom to a child?
- 7 Are there atoms in everything?
- 8 What do scientists call each kind of atom?
- 9 What do atoms do?
- 10 What is an atom 6th grade?
- 11 What are the 5 types of atoms?
- 12 What is meant by an atom being happy?
- 13 What is buzzing around the middle of an atom?
- 14 How do you describe an atom?
- 15 Is oxygen an atom?
- 16 How long do atoms last?
Atoms are the basic units of matter and the defining structure of elements. The term “atom” comes from the Greek word for indivisible, because it was once thought that atoms were the smallest things in the universe and could not be divided. … Atoms were created after the Big Bang 13.7 billion years ago.
What does the Greek meaning of atom mean?
FLATOW: A-T-O-M. … But when it comes to the word atom, we have to go to ancient Greece of 400 B.C. And there was a brilliant philosopher named Democritus, and he proposed the Greek word atomos, which means uncuttable. And so as he explained, all matter was eventually reducible to discrete, small particles or atomos.
What is the meaning of atom example?
Many atoms consist of a positively charged nucleus consisting of protons and neutrons surrounded by a cloud of electrons charged negatively. An atom is any particle of matter at its most basic level which contains at least one proton. Here are some examples of the atoms: hydrogen (H) neon (Ne).
What is an atom in simple words?
atom, smallest unit into which matter can be divided without the release of electrically charged particles. It also is the smallest unit of matter that has the characteristic properties of a chemical element. As such, the atom is the basic building block of chemistry.
Are humans made of atoms?
About 99 percent of your body is made up of atoms of hydrogen, carbon, nitrogen and oxygen. You also contain much smaller amounts of the other elements that are essential for life. … The very heavy elements in you were made in exploding stars. The size of an atom is governed by the average location of its electrons.
How do you explain an atom to a child?
The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons. Atoms fit together with other atoms to make up matter.
Are there atoms in everything?
(Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms. An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons.
What do scientists call each kind of atom?
Atoms are very small pieces of matter. There are many different types of atoms, each with its own name, mass and size. These different types of atoms are called chemical elements. The chemical elements are organized on the periodic table.
What do atoms do?
An atom is the smallest unit of matter that retains all of the chemical properties of an element. Atoms combine to form molecules, which then interact to form solids, gases, or liquids. For example, water is composed of hydrogen and oxygen atoms that have combined to form water molecules.
What is an atom 6th grade?
Atom- the smallest component of an element having the chemical properties of the element, consisting of a nucleus containing combinations of neutrons and protons and one or more electrons bound to the nucleus by electrical attraction. … Neutron – Located in the nucleus and have a no charge.
What are the 5 types of atoms?
Different Kinds of Atoms
- Description. Atoms are made of tiny particles called protons, neutrons and electrons. …
- Stable. Most atoms are stable. …
- Isotopes. Every atom is a chemical element, like hydrogen, iron or chlorine. …
- Radioactive. Some atoms have too many neutrons in the nucleus, which makes them unstable. …
- Ions. …
- Antimatter.
What is meant by an atom being happy?
We use a concept called “Happy Atoms.” We figure that most atoms want to be happy, just like you. The idea behind Happy Atoms is that atomic shells like to be full. That’s it. If you are an atom and you have a shell, you want your shell to be full. … These atoms like to give up their electrons.
What is buzzing around the middle of an atom?
1. Everything is made up of tiny particles called Proper proportioned giant atom model of science: The nucleus is the middle of the atom. … Buzzing around the outside of the nucleus are very small particles called The flow of electrons from one atom to another is called electricity.
How do you describe an atom?
An atom is a particle of matter that uniquely defines achemical element. An atom consists of a central nucleus that is usually surrounded by one or more electrons. … The nucleus is positively charged, and contains one or more relatively heavy particles known as protons and neutrons. A proton is positively charged.
Is oxygen an atom?
Oxygen is a chemical element – a substance that contains only one type of atom. Its official chemical symbol is O, and its atomic number is 8, which means that an oxygen atom has eight protons in its nucleus. Oxygen is a gas at room temperature and has no colour, smell or taste. Oxygen is found naturally as a molecule.
How long do atoms last?
Ultimately, even these stable atoms have a limit imposed by the lifetime of proton (>1025 years). Remember, though, that the best estimate of the present age of the universe is the much smaller number of 1010 years, so for all practical purposes, atoms are forever.